- Record: found
- Abstract: found
- Article: found
Myocardial Matrix Hydrogels Mitigate Negative Remodeling and Improve Function in Right Heart Failure Model
Read this article at
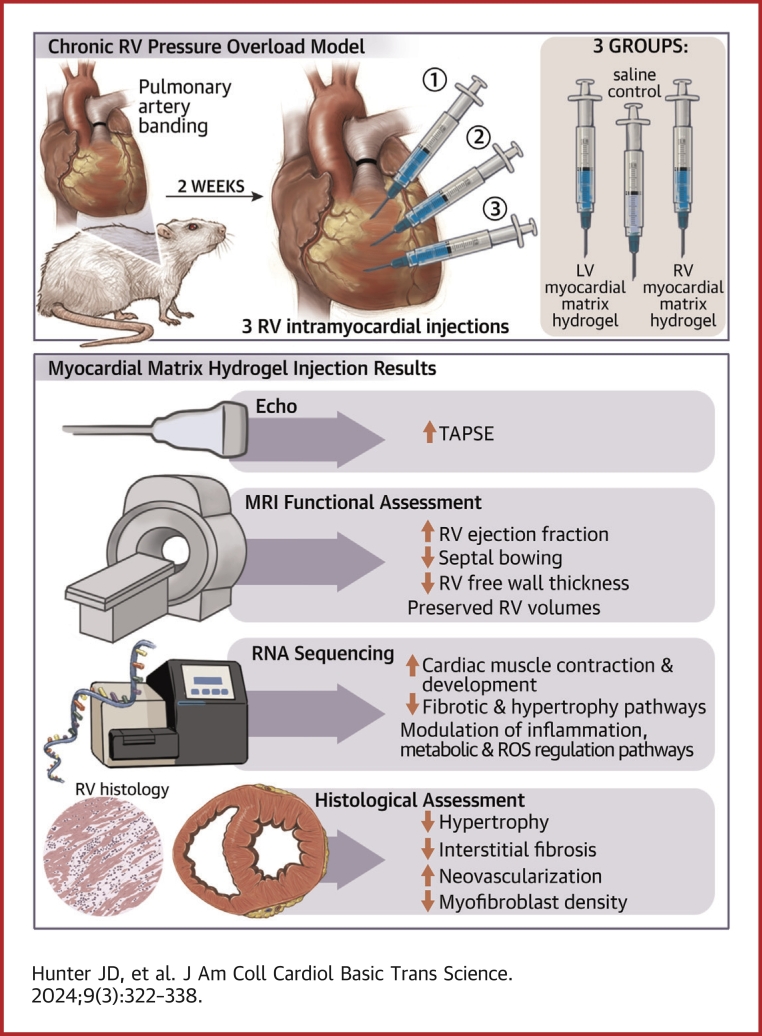
Visual Abstract
Highlights
-
•
LV- and RV-derived MM hydrogels improve RV contractility over time, preserve RV volumes, and reduce RV-free wall thickness.
-
•
LV-derived MM hydrogels improve RVEF.
-
•
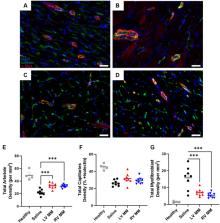
LV- and RV-derived MM hydrogels reduce hypertrophy and interstitial fibrosis, decrease myofibroblast density, and enhance neovascularization.
-
•
Although both MM hydrogels enhance gene expression related to neovascularization, contractility, and cardiac development, the RV-derived MM hydrogel enhances inflammatory and fibrotic gene expression, suggesting greater therapeutic benefit of the LV-derived MM hydrogel.
Summary
This study evaluates the effectiveness of myocardial matrix (MM) hydrogels in mitigating negative right ventricular (RV) remodeling in a rat model of RV heart failure. The goal was to assess whether a hydrogel derived from either the right or left ventricle could promote cardiac repair. Injured rat right ventricles were injected with either RV-or left ventricular–derived MM hydrogels. Both hydrogels improved RV function and morphology and reduced negative remodeling. This study supports the potential of injectable biomaterial therapies for treating RV heart failure.
Related collections
Most cited references80
- Record: found
- Abstract: found
- Article: not found
Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells.
- Record: found
- Abstract: found
- Article: not found
Extracellular matrix hydrogels from decellularized tissues: Structure and function
- Record: found
- Abstract: found
- Article: not found