- Record: found
- Abstract: found
- Article: found
Accelerated Degradation of Perfluorosulfonates and Perfluorocarboxylates by UV/Sulfite + Iodide: Reaction Mechanisms and System Efficiencies
Read this article at
Abstract

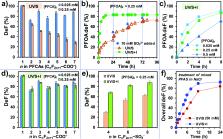
The addition of iodide (I –) in the UV/sulfite system (UV/S) significantly accelerated the reductive degradation of perfluorosulfonates (PFSAs, C n F 2 n+1 SO 3 –) and perfluorocarboxylates (PFCAs, C n F 2 n+1 COO –). Using the highly recalcitrant perfluorobutane sulfonate (C 4F 9SO 3 –) as a probe, we optimized the UV/sulfite + iodide system (UV/S + I) to degrade n = 1–7 PFCAs and n = 4, 6, 8 PFSAs. In general, the kinetics of per- and polyfluoroalkyl substance (PFAS) decay, defluorination, and transformation product formations in UV/S + I were up to three times faster than those in UV/S. Both systems achieve a similar maximum defluorination. The enhanced reaction rates and optimized photoreactor settings lowered the EE/O for PFCA degradation below 1.5 kW h m –3. The relatively high quantum yield of e aq – from I – made the availability of hydrated electrons (e aq –) in UV/S + I and UV/I two times greater than that in UV/S. Meanwhile, the rapid scavenging of reactive iodine species by SO 3 2– made the lifetime of e aq – in UV/S + I eight times longer than that in UV/I. The addition of I – also substantially enhanced SO 3 2– utilization in treating concentrated PFAS. The optimized UV/S + I system achieved >99.7% removal of most PFSAs and PFCAs and >90% overall defluorination in a synthetic solution of concentrated PFAS mixtures and NaCl. We extended the discussion over molecular transformation mechanisms, development of PFAS degradation technologies, and the fate of iodine species.
Abstract
Adding iodide in the UV/sulfite system significantly enhanced the reaction rate, energy efficiency, and chemical utilization for reductive defluorination of various per- and polyfluoroalkyl substance pollutants.
Related collections
Most cited references58
- Record: found
- Abstract: not found
- Article: not found
Critical Review of rate constants for reactions of hydrated electronsChemical Kinetic Data Base for Combustion Chemistry. Part 3: Propane
- Record: found
- Abstract: not found
- Article: not found
Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution
- Record: found
- Abstract: not found
- Article: not found