- Record: found
- Abstract: found
- Article: found
Efficacy and safety of ibuprofen gargle for postoperative pain after mandibular third molar extraction: A phase II, placebo‐controlled, double‐blind, randomized crossover trial
Read this article at
Abstract
Objective
This study was designed to evaluate the postoperative efficacy and safety of using an ibuprofen gargle as a pain management strategy for patients who have undergone mandibular third molar extraction. We also ensured that the quality of treatment was not compromised throughout the study.
Material and Methods
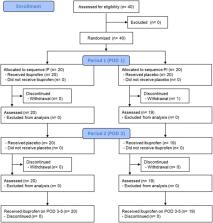
Patients were randomized in a 1:1 ratio into two groups: the ibuprofen–placebo (IP) group and the placebo–ibuprofen (PI) group. On postoperative Day (POD) 1, the IP group initiated ibuprofen administration, while the PI group started taking placebo. On POD 2, the IP group switched to using placebo, whereas the PI group switched to ibuprofen. From PODs 3–5, both groups were prescribed ibuprofen gargle. The primary endpoint was within‐subject visual analog scale (VAS) score before and 5 min after the first use of the ibuprofen or placebo gargle on PODs 1 and 2 (ΔVAS 5_ibuprofen − ΔVAS 5_placebo). The incidence and severity of adverse events were assessed using the Common Terminology Criteria for Adverse Events version 5.0 and a subjective rating scale.
Results
This study enrolled 40 patients. The within‐subject VAS 5 of the IP and PI groups were 1.25 ± 12.0 and −5.26 ± 8.93 mm, respectively. The treatment effect of ibuprofen gargle was −2.01 ± 10.62 mm ( p = .246). None of the patients in each group presented with serious adverse events or clinically significant complications (including dry sockets) after extraction. Transient adverse events, such as throat tingling and oral discomfort (grade 1), were observed in each group.
Related collections
Most cited references54
- Record: found
- Abstract: not found
- Article: not found
CONSORT 2010 statement: extension to randomised crossover trials
- Record: found
- Abstract: found
- Article: not found
Ibuprofen: pharmacology, efficacy and safety.
- Record: found
- Abstract: found
- Article: not found