- Record: found
- Abstract: found
- Article: not found
TiO 2/BiVO 4 Nanowire Heterostructure Photoanodes Based on Type II Band Alignment
Read this article at
Abstract

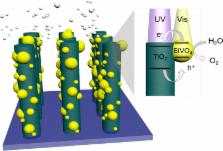
Metal oxides that absorb visible light are attractive for use as photoanodes in photoelectrosynthetic cells. However, their performance is often limited by poor charge carrier transport. We show that this problem can be addressed by using separate materials for light absorption and carrier transport. Here, we report a Ta:TiO 2|BiVO 4 nanowire photoanode, in which BiVO 4 acts as a visible light-absorber and Ta:TiO 2 acts as a high surface area electron conductor. Electrochemical and spectroscopic measurements provide experimental evidence for the type II band alignment necessary for favorable electron transfer from BiVO 4 to TiO 2. The host–guest nanowire architecture presented here allows for simultaneously high light absorption and carrier collection efficiency, with an onset of anodic photocurrent near 0.2 V vs RHE, and a photocurrent density of 2.1 mA/cm 2 at 1.23 V vs RHE.
Abstract
We report the use of Ta:TiO 2|BiVO 4 as a photoanode for use in solar water splitting cells. This host−guest system makes use of the favorable band alignment between the two semiconductors. The nanowire architecture allows for simultaneously high light absorption and carrier collection for efficient solar water oxidation.
Related collections
Most cited references49
- Record: found
- Abstract: not found
- Article: not found
Atomic layer deposition: an overview.
- Record: found
- Abstract: not found
- Article: not found
A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production
- Record: found
- Abstract: found
- Article: not found
