- Record: found
- Abstract: found
- Article: found
Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion
Read this article at
Summary
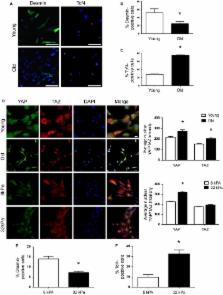
Age‐related declines in skeletal muscle regeneration have been attributed to muscle stem cell (MuSC) dysfunction. Aged MuSCs display a fibrogenic conversion, leading to fibrosis and impaired recovery after injury. Although studies have demonstrated the influence of in vitro substrate characteristics on stem cell fate, whether and how aging of the extracellular matrix (ECM) affects stem cell behavior has not been investigated. Here, we investigated the direct effect of the aged muscle ECM on MuSC lineage specification. Quantification of ECM topology and muscle mechanical properties reveals decreased collagen tortuosity and muscle stiffening with increasing age. Age‐related ECM alterations directly disrupt MuSC responses, and MuSCs seeded ex vivo onto decellularized ECM constructs derived from aged muscle display increased expression of fibrogenic markers and decreased myogenicity, compared to MuSCs seeded onto young ECM. This fibrogenic conversion is recapitulated in vitro when MuSCs are seeded directly onto matrices elaborated by aged fibroblasts. When compared to young fibroblasts, fibroblasts isolated from aged muscle display increased nuclear levels of the mechanosensors, Yes‐associated protein (YAP)/transcriptional coactivator with PDZ‐binding motif (TAZ), consistent with exposure to a stiff microenvironment in vivo. Accordingly, preconditioning of young fibroblasts by seeding them onto a substrate engineered to mimic the stiffness of aged muscle increases YAP/TAZ nuclear translocation and promotes secretion of a matrix that favors MuSC fibrogenesis. The findings here suggest that an age‐related increase in muscle stiffness drives YAP/TAZ‐mediated pathogenic expression of matricellular proteins by fibroblasts, ultimately disrupting MuSC fate.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Cellular mechanotransduction: putting all the pieces together again.
- Record: found
- Abstract: found
- Article: not found
Notch-mediated restoration of regenerative potential to aged muscle.
- Record: found
- Abstract: found
- Article: not found