- Record: found
- Abstract: found
- Article: found
Microtubule Organization in Striated Muscle Cells
Read this article at
Abstract
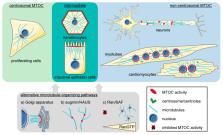
Distinctly organized microtubule networks contribute to the function of differentiated cell types such as neurons, epithelial cells, skeletal myotubes, and cardiomyocytes. In striated (i.e., skeletal and cardiac) muscle cells, the nuclear envelope acts as the dominant microtubule-organizing center (MTOC) and the function of the centrosome—the canonical MTOC of mammalian cells—is attenuated, a common feature of differentiated cell types. We summarize the mechanisms known to underlie MTOC formation at the nuclear envelope, discuss the significance of the nuclear envelope MTOC for muscle function and cell cycle progression, and outline potential mechanisms of centrosome attenuation.
Related collections
Most cited references225
- Record: found
- Abstract: found
- Article: not found
Skeletal muscle: a brief review of structure and function.
- Record: found
- Abstract: found
- Article: not found
The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response.
- Record: found
- Abstract: found
- Article: not found