- Record: found
- Abstract: found
- Article: found
Comparative Analysis of Pharmacodynamics in the C3HeB/FeJ Mouse Tuberculosis Model for DprE1 Inhibitors TBA-7371, PBTZ169, and OPC-167832
Read this article at
ABSTRACT
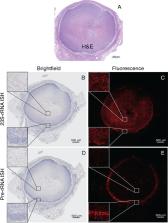
Multiple drug discovery initiatives for tuberculosis are currently ongoing to identify and develop new potent drugs with novel targets in order to shorten treatment duration. One of the drug classes with a new mode of action is DprE1 inhibitors targeting an essential process in cell wall synthesis of Mycobacterium tuberculosis. In this investigation, three DprE1 inhibitors currently in clinical trials, TBA-7371, PBTZ169, and OPC-167832, were evaluated side-by-side as single agents in the C3HeB/FeJ mouse model presenting with caseous necrotic pulmonary lesions upon tuberculosis infection. The goal was to confirm the efficacy of the DprE1 inhibitors in a mouse tuberculosis model with advanced pulmonary pathology and perform comprehensive analysis of plasma, lung, and lesion-centric drug levels to establish pharmacokinetic-pharmacodynamic (PK-PD) parameters predicting efficacy at the site of infection. Results showed significant efficacy for all three DprE1 inhibitors in the C3HeB/FeJ mouse model after 2 months of treatment. Superior efficacy was observed for OPC-167832 even at low-dose levels, which can be attributed to its low MIC, favorable distribution, and sustained retention above the MIC throughout the dosing interval in caseous necrotic lesions, where the majority of bacteria reside in C3HeB/FeJ mice. These results support further progression of the three drug candidates through clinical development for tuberculosis treatment.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study
- Record: found
- Abstract: found
- Article: not found
An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence.
- Record: found
- Abstract: found
- Article: not found