- Record: found
- Abstract: found
- Article: found
Focal adhesion kinase overexpression and its impact on human osteosarcoma
Read this article at
Abstract
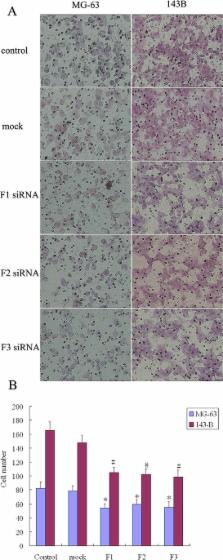
Focal adhesion kinase (FAK) has been implicated in tumorigenesis in various malignancies. We sought to examine the expression patterns of FAK and the activated form, phosphorylated FAK (pFAK), in human osteosarcoma and to investigate the correlation of FAK expression with clinicopathologic parameters and prognosis. In addition, the functional consequence of manipulating the FAK protein level was investigated in human osteosarcoma cell lines. Immunohistochemical staining was used to detect FAK and pFAK in pathologic archived materials from 113 patients with primary osteosarcoma. Kaplan-Meier survival and Cox regression analyses were performed to evaluate the prognoses. The role of FAK in the cytological behavior of MG63 and 143B human osteosarcoma cell lines was studied via FAK protein knock down with siRNA. Cell proliferation, migration, invasiveness and apoptosis were assessed using the CCK8, Transwell and Annexin V/PI staining methods. Both FAK and pFAK were overexpressed in osteosarcoma. There were significant differences in overall survival between the FAK-/pFAK- and FAK+/pFAK- groups ( P = 0.016), the FAK+/pFAK- and FAK+/pFAK+ groups ( P = 0.012) and the FAK-/pFAK- and FAK+/pFAK+ groups ( P < 0.001). There were similar differences in metastasis-free survival between groups. The Cox proportional hazards analysis showed that the FAK expression profile was an independent indicator of both overall and metastasis-free survival. siRNA-based knockdown of FAK not only dramatically reduced the migration and invasion of MG63 and 143B cells, but also had a distinct effect on osteosarcoma cell proliferation and apoptosis. These results collectively suggest that FAK overexpression and phosphorylation might predict more aggressive biologic behavior in osteosarcoma and may be an independent predictor of poor prognosis.
Related collections
Most cited references73
- Record: found
- Abstract: found
- Article: not found
Disruption of epithelial cell-matrix interactions induces apoptosis
- Record: found
- Abstract: found
- Article: not found
Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia.
- Record: found
- Abstract: found
- Article: not found