- Record: found
- Abstract: found
- Article: found
Amyloid Precursor Protein and Proinflammatory Changes Are Regulated in Brain and Adipose Tissue in a Murine Model of High Fat Diet-Induced Obesity
Read this article at
Abstract
Background
Middle age obesity is recognized as a risk factor for Alzheimer's disease (AD) although a mechanistic linkage remains unclear. Based upon the fact that obese adipose tissue and AD brains are both areas of proinflammatory change, a possible common event is chronic inflammation. Since an autosomal dominant form of AD is associated with mutations in the gene coding for the ubiquitously expressed transmembrane protein, amyloid precursor protein (APP) and recent evidence demonstrates increased APP levels in adipose tissue during obesity it is feasible that APP serves some function in both disease conditions.
Methodology/Principal Findings
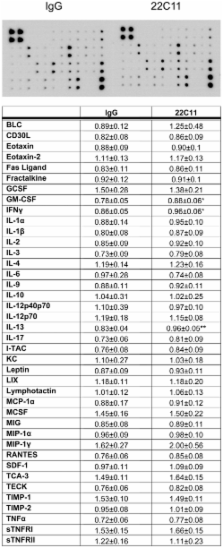
To determine whether diet-induced obesity produced proinflammatory changes and altered APP expression in brain versus adipose tissue, 6 week old C57BL6/J mice were maintained on a control or high fat diet for 22 weeks. Protein levels and cell-specific APP expression along with markers of inflammation and immune cell activation were compared between hippocampus, abdominal subcutaneous fat and visceral pericardial fat. APP stimulation-dependent changes in macrophage and adipocyte culture phenotype were examined for comparison to the in vivo changes.
Conclusions/Significance
Adipose tissue and brain from high fat diet fed animals demonstrated increased TNF-α and microglial and macrophage activation. Both brains and adipose tissue also had elevated APP levels localizing to neurons and macrophage/adipocytes, respectively. APP agonist antibody stimulation of macrophage cultures increased specific cytokine secretion with no obvious effects on adipocyte culture phenotype. These data support the hypothesis that high fat diet-dependent obesity results in concomitant pro-inflammatory changes in brain and adipose tissue that is characterized, in part, by increased levels of APP that may be contributing specifically to inflammatory changes that occur.
Related collections
Most cited references91
- Record: found
- Abstract: found
- Article: not found
MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity.
- Record: found
- Abstract: found
- Article: not found
Activation of microglial cells by beta-amyloid protein and interferon-gamma.
- Record: found
- Abstract: found
- Article: not found