- Record: found
- Abstract: found
- Article: found
Retinal blood flow association with age and weight in infants at risk for retinopathy of prematurity
Read this article at
Abstract
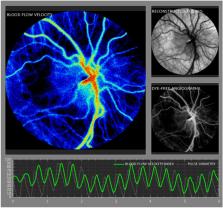
This prospective study evaluated the relationship between laser speckle contrast imaging (LSCI) ocular blood flow velocity (BFV) and five birth parameters: gestational age (GA), postmenstrual age (PMA) and chronological age (CA) at the time of measurement, birth weight (BW), and current weight (CW) in preterm neonates at risk for retinopathy of prematurity (ROP). 38 Neonates with BW < 2 kg, GA < 32 weeks, and PMA between 27 and 47 weeks underwent 91 LSCI sessions. Correlation tests and regression analysis were performed to quantify relationships between birth parameters and ocular BFV. Mean ocular BFV index in this cohort was 8.8 +/− 4.0 IU. BFV positively correlated with PMA (r = 0.3, p = 0.01), CA (r = 0.3, p = 0.005), and CW (r = 0.3, p = 0.02). BFV did not correlate with GA nor BW (r = − 0.2 and r = − 0.05, p > 0.05). Regression analysis with mixed models demonstrated that BFV increased by 1.2 for every kilogram of CW, by 0.34 for every week of CA, and by 0.36 for every week of PMA ( p = 0.03, 0.004, 0.007, respectively). Our findings indicate that increased age and weight are associated with increased ocular BFV measured using LSCI in premature infants. Future studies investigating the associations between ocular BFV and ROP clinical severity must control for age and/or weight of the infant.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Retinopathy of prematurity.
- Record: found
- Abstract: found
- Article: not found
Screening Examination of Premature Infants for Retinopathy of Prematurity
- Record: found
- Abstract: found
- Article: not found