- Record: found
- Abstract: found
- Article: found
Efficacy of acupuncture at three nasal acupoints plus acupoint application for perennial allergic rhinitis: A multicenter, randomized controlled trial protocol
Read this article at
Abstract
Background
Many studies have shown the potential therapeutic effect of acupuncture on allergic rhinitis. Most of these studies were limited by low-quality evidence. Preliminary experiments showed that the use of acupuncture at three nasal acupoints plus acupoint application (AAP) achieves a more persistent effect in the treatment of perennial allergic rhinitis than acupuncture alone. In this study, a multicenter, single-blind, randomized controlled trial will be performed, in which acupuncture at nonmeridian acupoints and sham AAP will be used as the control group to evaluate the effect of AAP through long-term observation.
Methods
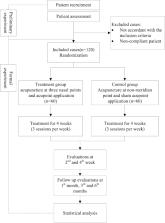
The trial is designed on the basis of the Consolidated Standards of Reporting Trials 2010 guidelines and Standards for Reporting Interventions in Controlled Trials of Acupuncture. A total of 120 participants with perennial allergic rhinitis will be randomly assigned to a treatment or control group. A specially appointed investigator will be in charge of randomization. The participants in the treatment group will be treated with acupuncture at EX-HN3, LI20, and EX-HN8 thrice per week for a total of 12 sessions. In addition, they will undergo AAP at DU14, BL13, EX-BI, and RN22. The participants in the control group will be treated with sham AAP. The primary outcome will be the change in the Total Nasal Symptom Score from baseline to the completion of 4-week treatment. Secondary outcomes include changes in visual analog scale and total non-nasal symptom scores from baseline to the second and fourth weeks of treatment, as well as 1, 3, and 6 months after the completion of treatment. Peripheral blood IL-4, IL-5, IL-6, IL-8, and IL-10 levels will be measured, and any side effects related to treatment will be observed and recorded.
Discussion
It is expected that this randomized clinical trial will provide evidence to determine the effects of AAP compared with acupuncture at nonmeridian acupoints and sham AAP, particularly the long-term effect. These findings will help improve the clinical application of this technique.
Trial registration
Acupuncture-Moxibustion Clinical Trial Registry AMCTR-ICR-18000179. Registered on 12 April 2018.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Long-term clinical efficacy of grass-pollen immunotherapy.
- Record: found
- Abstract: found
- Article: not found
Clinical practice guideline: Allergic rhinitis.
- Record: found
- Abstract: found
- Article: not found