- Record: found
- Abstract: found
- Article: not found
Immunomodulatory therapy using a pediatric dialysis system ameliorates septic shock in miniature pigs
Read this article at
Abstract
Background:
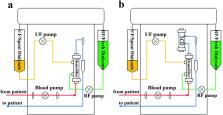
Application of the immunomodulatory Selective Cytopheretic Device (SCD) to enhance renal replacement therapy and improve outcomes of acute kidney injury in pediatric patients is impeded by safety concerns. Therapy using a pediatric hemodialysis system could overcome these limitations.
Methods:
Yucatan minipigs (8–15kg) with induced septic shock underwent continuous hemodiafiltration with the CARPEDIEM pediatric hemodialysis system using regional citrate anticoagulation (RCA) with or without SCD (n=5 per group). Circuit function plus hemodynamic and hematologic parameters were assessed for 6h.
Results:
SCD was readily integrated into the CARPEDIEM ™ system and treatment delivered for 6 hours without interference with pump operation. SCD treated pigs maintained higher blood pressure (p=0.009) commensurate with lesser degree of lactic acidosis (p=0.008) compared to pigs only receiving hemodiafiltration. Renal failure occurred in untreated pigs while urine output was sustained with SCD therapy. Neutrophil activation levels and ssSOFA scores at 6 hour trended lower in the SCD treated cohort.
Conclusions:
SCD therapy under RCA was safely administered using the CARPEDIEM ™ pediatric hemodialysis system for up to 6 hours and no circuit compatibility issues were identified. Sepsis progression and organ dysfunction was diminished with SCD treatment in this model supportive of therapeutic benefit of this immunomodulatory therapy.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Swine as models in biomedical research and toxicology testing.
- Record: found
- Abstract: not found
- Article: not found
The pathophysiology and treatment of sepsis.
- Record: found
- Abstract: found
- Article: not found
