- Record: found
- Abstract: found
- Article: found
Galectin‐3, a Biomarker Linking Oxidative Stress and Inflammation With the Clinical Outcomes of Patients With Atherothrombosis
Read this article at
Abstract
Background
Galectin‐3 (Gal‐3) participates in different mechanisms involved in atherothrombosis, such as inflammation, proliferation, or macrophage chemotaxis. Thus, there have been committed intensive efforts to elucidate the function of Gal‐3 in cardiovascular (CV) diseases. The role of Gal‐3 as a circulating biomarker has been demonstrated in patients with heart failure, but its importance as a biomarker in atherothrombosis is still unknown.
Methods and Results
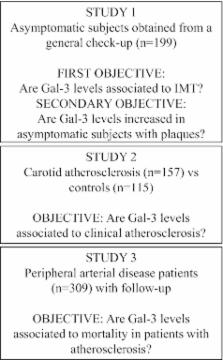
Because Gal‐3 is involved in monocyte‐to‐macrophage transition, we used fresh isolated monocytes and the in vitro model of macrophage differentiation of THP‐1 cells stimulated with phorbol myristate acetate (PMA). Gal‐3 release is increased by PMA in human monocytes and macrophages, a process involving exosomes and regulated by reactive oxygen species/NADPH oxidase activity. In asymptomatic subjects (n=199), Gal‐3 plasma levels are correlated with NADPH oxidase activity in peripheral blood mononuclear cells ( r=0.476; P<0.001) and carotid intima‐media thickness ( r=0.438; P<0.001), a surrogate marker of atherosclerosis. Accordingly, Gal‐3 plasma concentrations are increased in patients with carotid atherosclerosis (n=158), compared to control subjects (n=115; 14.3 [10.7 to 16.9] vs. 10.4 [8.6 to 12.5] ng/mL; P<0.001). Finally, on a 5‐year follow‐up study in patients with peripheral artery disease, Gal‐3 concentrations are significantly and independently associated with an increased risk for CV mortality (hazard ratio=2.24, 95% confidence interval: 1.06 to 4.73, P<0.05).
Conclusions
Gal‐3 extracellular levels could reflect key underlying mechanisms involved in atherosclerosis etiology, development, and plaque rupture, such as inflammation, infiltration of circulating cells and oxidative stress. Moreover, circulating Gal‐3 concentrations are associated with clinical outcomes in patients with atherothrombosis.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles.
- Record: found
- Abstract: found
- Article: not found
Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction.
- Record: found
- Abstract: found
- Article: not found