- Record: found
- Abstract: found
- Article: found
Prostate specific membrane antigen (PSMA) expression in primary gliomas and breast cancer brain metastases
Read this article at
Abstract
Background
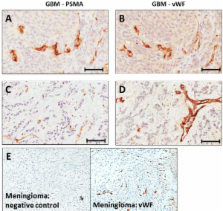
Primary and secondary brain cancers are highly treatment resistant, and their marked angiogenesis attracts interest as a potential therapeutic target. Recent observations reveal that the microvascular endothelium of primary high-grade gliomas expresses prostate specific membrane antigen (PSMA). Breast cancers express PSMA and they frequently form secondary brain tumors. Hence we report here our pilot study addressing the feasibility of PSMA targeting in brain and metastatic breast tumors, by examining PSMA levels in all glioma grades (19 patients) and in breast cancer brain metastases (5 patients).
Methods
Tumor specimens were acquired from archival material and normal brain tissues from autopsies. Tissue were stained and probed for PSMA, and the expression levels imaged and quantified using automated hardware and software. PSMA staining intensities of glioma subtypes, breast tumors, and breast tumor brain metastases were compared statistically versus normals.
Results
Normal brain microvessels (4 autopsies) did not stain for PSMA, while a small proportion (<5%) of healthy neurons stained, and were surrounded by an intact blood brain barrier. Tumor microvessels of the highly angiogenic grade IV gliomas showed intense PSMA staining which varied between patients and was significantly higher (p < 0.05) than normal brain. Grade I gliomas showed moderate vessel staining, while grade II and III gliomas had no vessel staining, but a few (<2%) of the tumor cells stained. Both primary breast cancer tissues and the associated brain metastases exhibited vascular PSMA staining, although the intensity of staining was generally less for the metastatic lesions.
Conclusions
Our results align with and extend previous data showing PSMA expression in blood vessels of gliomas and breast cancer brain metastases. These results provide a rationale for more comprehensive studies to explore PSMA targeted agents for treating secondary brain tumors with PSMA expressing vasculature. Moreover, given that PSMA participates in angiogenesis, cell signaling, tumor survival, and invasion, characterizing its expression may help guide later investigations of the poorly understood process of low grade glioma progression to glioblastoma.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Angiogenesis in brain tumours.
- Record: found
- Abstract: found
- Article: not found
Brain metastases: epidemiology and pathophysiology.
- Record: found
- Abstract: found
- Article: not found