- Record: found
- Abstract: found
- Article: found
S1PR1 regulates the switch of two angiogenic modes by VE-cadherin phosphorylation in breast cancer
Read this article at
Abstract
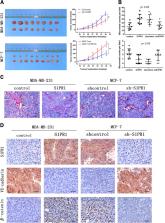
Angiogenesis in solid tumors is divided into two modes: endothelium-dependent vessel (EDV) and vasculogenic mimicry (VM). Sphingosine-1-phosphate receptor 1 (S1PR1) plays a vital role on EDV in a variety of human tumors. However, the relationship between S1PR1 and VM is not clear. The aim of this study is to investigate S1PR1 on the regulation of EDV and mimicry formation in breast cancer. Here we show that S1PR1 phosphorylates the complex of VE-cadherin to regulate the switch of EDV and mimicry formation. Suppression of S1PR1 impairs EDV, but contributes to the generation of VM, invasion, and metastasis in vivo and vitro. By inhibiting RhoA activation, the S1PR1/VE-cadherin signaling is blocked. S1PR1 controls VE-cadherin expression and EDV via RhoA activation. Moreover, the low expression of S1PR1 correlates with VM and poor prognosis in breast cancer patient. The results show that S1PR1 regulated RhoA activation to accelerate VE-cadherin phosphorylation (Y731), leading to increased EDV and reduced VM in breast cancer. S1PR1 may provide a new thinking direction for antiangiogenic therapy for patients with breast cancer.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy.
- Record: found
- Abstract: found
- Article: not found
Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation.
- Record: found
- Abstract: found
- Article: not found