- Record: found
- Abstract: found
- Article: found
The Future of Bronchopulmonary Dysplasia: Emerging Pathophysiological Concepts and Potential New Avenues of Treatment
Read this article at
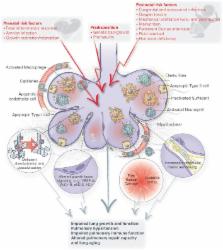
Abstract
Yearly more than 15 million babies are born premature (<37 weeks gestational age), accounting for more than 1 in 10 births worldwide. Lung injury caused by maternal chorioamnionitis or preeclampsia, postnatal ventilation, hyperoxia, or inflammation can lead to the development of bronchopulmonary dysplasia (BPD), one of the most common adverse outcomes in these preterm neonates. BPD patients have an arrest in alveolar and microvascular development and more frequently develop asthma and early-onset emphysema as they age. Understanding how the alveoli develop, and repair, and regenerate after injury is critical for the development of therapies, as unfortunately there is still no cure for BPD. In this review, we aim to provide an overview of emerging new concepts in the understanding of perinatal lung development and injury from a molecular and cellular point of view and how this is paving the way for new therapeutic options to prevent or treat BPD, as well as a reflection on current treatment procedures.
Related collections
Most cited references204
- Record: found
- Abstract: found
- Article: not found
Preparing for the first breath: genetic and cellular mechanisms in lung development.
- Record: found
- Abstract: found
- Article: not found
Caffeine therapy for apnea of prematurity.
- Record: found
- Abstract: not found
- Article: not found