- Record: found
- Abstract: found
- Article: found
Immunometabolic Pathways in BCG-Induced Trained Immunity
Read this article at
Summary
The protective effects of the tuberculosis vaccine Bacillus Calmette-Guerin (BCG) on unrelated infections are thought to be mediated by long-term metabolic changes and chromatin remodeling through histone modifications in innate immune cells such as monocytes, a process termed trained immunity. Here, we show that BCG induction of trained immunity in monocytes is accompanied by a strong increase in glycolysis and, to a lesser extent, glutamine metabolism, both in an in-vitro model and after vaccination of mice and humans. Pharmacological and genetic modulation of rate-limiting glycolysis enzymes inhibits trained immunity, changes that are reflected by the effects on the histone marks (H3K4me3 and H3K9me3) underlying BCG-induced trained immunity. These data demonstrate that a shift of the glucose metabolism toward glycolysis is crucial for the induction of the histone modifications and functional changes underlying BCG-induced trained immunity. The identification of these pathways may be a first step toward vaccines that combine immunological and metabolic stimulation.
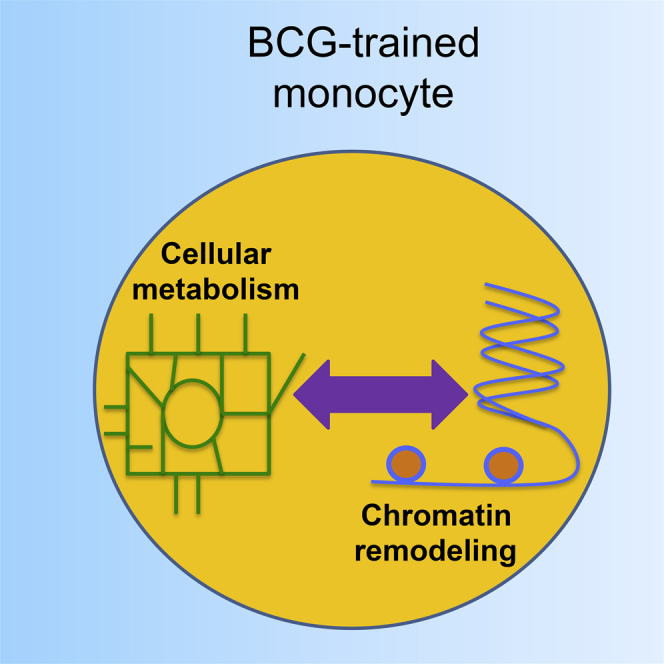
Graphical Abstract
Highlights
-
•
Cellular metabolism undergoes major shifts in BCG-trained monocytes
-
•
The Akt-mTOR signaling pathway is essential for these shifts in metabolism
-
•
Induction of glucose and glutamine metabolism are crucial in trained immunity
-
•
The metabolic changes are the result of rewiring of chromatin modifications
Abstract
Arts et al. found that glycolysis and glutamine metabolism, regulated by the Akt-mTOR pathway, are central mediators for the induction of trained immunity by BCG in monocytes. They show that metabolic changes are dependent on changes in histone modifications, which are influenced by metabolic changes.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity.
- Record: found
- Abstract: found
- Article: not found
Fueling immunity: insights into metabolism and lymphocyte function.
- Record: found
- Abstract: found
- Article: not found