- Record: found
- Abstract: found
- Article: found
Enterohemorrhagic Escherichia coli Reduces Mucus and Intermicrovillar Bridges in Human Stem Cell-Derived Colonoids
Read this article at
Abstract
Background & Aims
Enterohemorrhagic Escherichia coli (EHEC) causes over 70,000 episodes of foodborne diarrhea annually in the United States. The early sequence of events that precede life-threatening hemorrhagic colitis and hemolytic uremic syndrome is not fully understood due to the initial asymptomatic phase of the disease and the lack of a suitable animal model. We determined the initial molecular events in the interaction between EHEC and human colonic epithelium.
Methods
Human colonoids derived from adult proximal colonic stem cells were developed into monolayers to study EHEC-epithelial interactions. Monolayer confluency and differentiation were monitored by transepithelial electrical resistance measurements. The monolayers were apically infected with EHEC, and the progression of epithelial damage over time was assessed using biochemical and imaging approaches.
Results
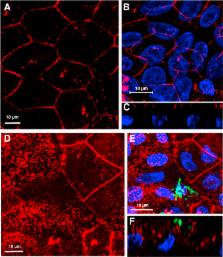
Human colonoid cultures recapitulate the differential protein expression patterns characteristic of the crypt and surface colonocytes. Mucus-producing differentiated colonoid monolayers are preferentially colonized by EHEC. Upon colonization, EHEC forms characteristic attaching and effacing lesions on the apical surface of colonoid monolayers. Mucin 2, a main component of colonic mucus, and protocadherin 24 (PCDH24), a microvillar resident protein, are targeted by EHEC at early stages of infection. The EHEC-secreted serine protease EspP initiates brush border damage through PCDH24 reduction.
Conclusions
Human colonoid monolayers are a relevant pathophysiologic model that allow the study of early molecular events during enteric infections. Colonoid monolayers provide access to both apical and basolateral surfaces, thus providing an advantage over three-dimensional cultures to study host–pathogen interactions in a controllable and tractable manner. EHEC reduces colonic mucus and affects the brush border cytoskeleton in the absence of commensal bacteria.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays.
- Record: found
- Abstract: found
- Article: not found
Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors.
- Record: found
- Abstract: found
- Article: not found