- Record: found
- Abstract: found
- Article: found
Physical Exercise and Alzheimer’s Disease: Effects on Pathophysiological Molecular Pathways of the Disease
Read this article at
Abstract
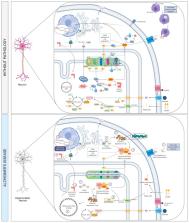
Alzheimer’s disease (AD), the most common form of neurodegenerative dementia in adults worldwide, is a multifactorial and heterogeneous disorder characterized by the interaction of genetic and epigenetic factors and the dysregulation of numerous intracellular signaling and cellular/molecular pathways. The introduction of the systems biology framework is revolutionizing the study of complex diseases by allowing the identification and integration of cellular/molecular pathways and networks of interaction. Here, we reviewed the relationship between physical activity and the next pathophysiological processes involved in the risk of developing AD, based on some crucial molecular pathways and biological process dysregulated in AD: (1) Immune system and inflammation; (2) Endothelial function and cerebrovascular insufficiency; (3) Apoptosis and cell death; (4) Intercellular communication; (5) Metabolism, oxidative stress and neurotoxicity; (6) DNA damage and repair; (7) Cytoskeleton and membrane proteins; (8) Synaptic plasticity. Moreover, we highlighted the increasingly relevant role played by advanced neuroimaging technologies, including structural/functional magnetic resonance imaging, diffusion tensor imaging, and arterial spin labelling, in exploring the link between AD and physical exercise. Regular physical exercise seems to have a protective effect against AD by inhibiting different pathophysiological molecular pathways implicated in AD.
Related collections
Most cited references204
- Record: found
- Abstract: found
- Article: not found
The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics.
- Record: found
- Abstract: found
- Article: found
Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease.
- Record: found
- Abstract: found
- Article: not found