- Record: found
- Abstract: found
- Article: not found
Polo-Like Kinase 1 Directs Assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF Complex to Initiate Cleavage Furrow Formation
Read this article at
Abstract
Polo-like kinase 1 promotes assembly of the contractile ring that divides a cell in two by creating a docking site for the RhoA activator Ect2 on the Cyk-4-containing centralspindlin complex at the midzone of the mitotic spindle.
Abstract
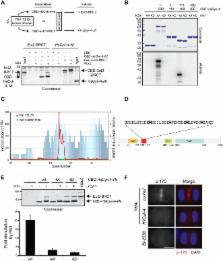
To complete cell division with high fidelity, cytokinesis must be coordinated with chromosome segregation. Mammalian Polo-like kinase 1, Plk1, may function as a critical link because it is required for chromosome segregation and establishment of the cleavage plane following anaphase onset. A central spindle–localized pool of the RhoGEF Ect2 promotes activation of the small GTPase RhoA, which drives contractile ring assembly at the equatorial cortex. Here, we have investigated how Plk1 promotes the central spindle recruitment of Ect2. Plk1 phosphorylates the noncatalytic N terminus of the RhoGAP HsCyk-4 at the central spindle, creating a phospho-epitope recognized by the BRCA1 C-terminal (BRCT) repeats of Ect2. Failure to phosphorylate HsCyk-4 blocks Ect2 recruitment to the central spindle and the subsequent induction of furrowing. Microtubules, as well as the microtubule-associated protein (MAP) Prc1, facilitate Plk1 phosphorylation of HsCyk-4. Characterization of a phosphomimetic version of HsCyk-4 indicates that Plk1 promotes Ect2 recruitment through multiple targets. Collectively, our data reveal that formation of the HsCyk-4-Ect2 complex is subject to multiple layers of regulation to ensure that RhoA activation occurs between the segregated sister chromatids during anaphase.
Author Summary
The plane of cell division in animal cells is determined by the position of the mitotic spindle during early anaphase, but the molecular signaling that leads to proper formation of the division plane is not fully understood. The actin- and myosin-rich contractile ring, which physically divides a cell in two, localizes to the presumptive division plane through the local activation of a molecular switch protein, RhoA. RhoA is activated by Ect2, which binds to the protein complex centralspindlin found on microtubules in the vicinity of the division plane (the midzone microtubules). One critical component of centralspindlin is Cyk-4, a putative negative regulator of RhoA. Here, we have analyzed the mechanisms that are responsible for targeting the RhoA activator Ect2 to the midzone microtubules. We show that Polo-like kinase 1 (Plk1), in part through the microtubule-associated protein Prc1, phosphorylates Cyk-4. Ect2 binds to phosphorylated Cyk-4 and is then able to activate RhoA and induce proper formation of the contractile ring. Our study therefore has elucidated important details of the signaling cascade in animal cells that ensures proper division-plane formation.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo.
- Record: found
- Abstract: found
- Article: not found
The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain.
- Record: found
- Abstract: found
- Article: not found
