- Record: found
- Abstract: found
- Article: found
Neuronal Redevelopment and the Regeneration of Neuromodulatory Axons in the Adult Mammalian Central Nervous System
Read this article at
Abstract
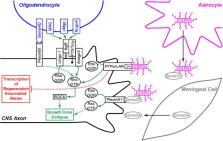
One reason that many central nervous system injuries, including those arising from traumatic brain injury, spinal cord injury, and stroke, have limited recovery of function is that neurons within the adult mammalian CNS lack the ability to regenerate their axons following trauma. This stands in contrast to neurons of the adult mammalian peripheral nervous system (PNS). New evidence, provided by single-cell expression profiling, suggests that, following injury, both mammalian central and peripheral neurons can revert to an embryonic-like growth state which is permissive for axon regeneration. This “redevelopment” strategy could both facilitate a damage response necessary to isolate and repair the acute damage from injury and provide the intracellular machinery necessary for axon regrowth. Interestingly, serotonin neurons of the rostral group of raphe nuclei, which project their axons into the forebrain, display a robust ability to regenerate their axons unaided, counter to the widely held view that CNS axons cannot regenerate without experimental intervention after injury. Furthermore, initial evidence suggests that norepinephrine neurons within the locus coeruleus possess similar regenerative abilities. Several morphological characteristics of serotonin axon regeneration in adult mammals, observable using longitudinal in vivo imaging, are distinct from the known characteristics of unaided peripheral nerve regeneration, or of the regeneration seen in the spinal cord and optic nerve that occurs with experimental intervention. These results suggest that there is an alternative CNS program for axon regeneration that likely differs from that displayed by the PNS.
Related collections
Most cited references140
- Record: found
- Abstract: found
- Article: not found
Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway.
- Record: found
- Abstract: found
- Article: not found
Astrocyte scar formation aids central nervous system axon regeneration.
- Record: found
- Abstract: found
- Article: not found