- Record: found
- Abstract: found
- Article: found
Protective Effects of Licochalcone A Ameliorates Obesity and Non-Alcoholic Fatty Liver Disease Via Promotion of the Sirt-1/AMPK Pathway in Mice Fed a High-Fat Diet
Read this article at
Abstract
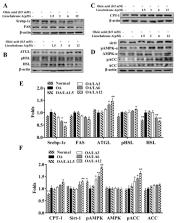
Licochalcone A is a chalcone isolated from Glycyrrhiza uralensis. It showed anti-tumor and anti-inflammatory properties in mice with acute lung injuries and regulated lipid metabolism through the activation of AMP-activated protein kinase (AMPK) in hepatocytes. However, the effects of licochalcone A on reducing weight gain and improving nonalcoholic fatty liver disease (NAFLD) are unclear. Thus, the present study investigated whether licochalcone A ameliorated weight loss and lipid metabolism in the liver of high-fat diet (HFD)-induced obese mice. Male C57BL/6 mice were fed an HFD to induce obesity and NAFLD, and then were injected intraperitoneally with licochalcone A. In another experiment, a fatty liver cell model was established by incubating HepG2 hepatocytes with oleic acid and treating the cells with licochalcone A to evaluate lipid metabolism. Our results demonstrated that HFD-induced obese mice treated with licochalcone A had decreased body weight as well as inguinal and epididymal adipose tissue weights compared with HFD-treated mice. Licochalcone A also ameliorated hepatocyte steatosis and decreased liver tissue weight and lipid droplet accumulation in liver tissue. We also found that licochalcone A significantly regulated serum triglycerides, low-density lipoprotein, and free fatty acids, and decreased the fasting blood glucose value. Furthermore, in vivo and in vitro, licochalcone A significantly decreased expression of the transcription factor of lipogenesis and fatty acid synthase. Licochalcone A activated the sirt-1/AMPK pathway to reduce fatty acid chain synthesis and increased lipolysis and β-oxidation in hepatocytes. Licochalcone A can potentially ameliorate obesity and NAFLD in mice via activation of the sirt1/AMPK pathway.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Transcriptional regulation of hepatic lipogenesis.

- Record: found
- Abstract: found
- Article: found
Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments
- Record: found
- Abstract: found
- Article: not found