- Record: found
- Abstract: found
- Article: found
Ferroptosis Enhanced Diabetic Renal Tubular Injury via HIF-1α/HO-1 Pathway in db/db Mice
Read this article at
Abstract
Background
Ferroptosis is a recently identified iron-dependent form of cell death as a result of increased reactive oxygen species (ROS) and lipid peroxidation. In this study, we investigated whether ferroptosis aggravated diabetic nephropathy (DN) and damaged renal tubules through hypoxia-inducible factor (HIF)-1α/heme oxygenase (HO)-1 pathway in db/db mice.
Methods
Db/db mice were administered with or without ferroptosis inhibitor Ferrostatin-1 treatment, and were compared with db/m mice.
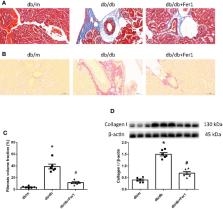
Results
Db/db mice showed higher urinary albumin-to-creatinine ratio (UACR) than db/m mice, and Ferrostatin-1 reduced UACR in db/db mice. Db/db mice presented higher kidney injury molecular-1 and neutrophil gelatinase-associated lipocalin in kidneys and urine compared to db/m mice, with renal tubular basement membranes folding and faulting. However, these changes were ameliorated in db/db mice after Ferrostatin-1 treatment. Fibrosis area and collagen I were promoted in db/db mouse kidneys as compared to db/m mouse kidneys, which was alleviated by Ferrostatin-1 in db/db mouse kidneys. HIF-1α and HO-1 were increased in db/db mouse kidneys compared with db/m mouse kidneys, and Ferrostatin-1 decreased HIF-1α and HO-1 in db/db mouse kidneys. Iron content was elevated in db/db mouse renal tubules compared with db/m mouse renal tubules, and was relieved in renal tubules of db/db mice after Ferrostatin-1 treatment. Ferritin was increased in db/db mouse kidneys compared with db/m mouse kidneys, but Ferrostatin-1 reduced ferritin in kidneys of db/db mice. Diabetes accelerated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-derived ROS formation in mouse kidneys, but Ferrostatin-1 prevented ROS formation derived by NADPH oxidases in db/db mouse kidneys. The increased malondialdehyde (MDA) and the decreased superoxide dismutase (SOD), catalase (CAT), glutathione peroxidases (GSH-Px) were detected in db/db mouse kidneys compared to db/m mouse kidneys, whereas Ferrostatin-1 suppressed MDA and elevated SOD, CAT, and GSH-Px in db/db mouse kidneys. Glutathione peroxidase 4 was lower in db/db mouse kidneys than db/m mouse kidneys, and was exacerbated by Ferrostatin-1 in kidneys of db/db mice.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice.

- Record: found
- Abstract: found
- Article: found
Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal
- Record: found
- Abstract: found
- Article: found