- Record: found
- Abstract: found
- Article: found
Monitoring of malaria parasite resistance to chloroquine and sulphadoxine-pyrimethamine in the Solomon Islands by DNA microarray technology
Read this article at
Abstract
Background
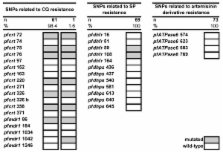
Little information is available on resistance to anti-malarial drugs in the Solomon Islands (SI). The analysis of single nucleotide polymorphisms (SNPs) in drug resistance associated parasite genes is a potential alternative to classical time- and resource-consuming in vivo studies to monitor drug resistance. Mutations in pfmdr1 and pfcrt were shown to indicate chloroquine (CQ) resistance, mutations in pfdhfr and pfdhps indicate sulphadoxine-pyrimethamine (SP) resistance, and mutations in pfATPase6 indicate resistance to artemisinin derivatives.
Methods
The relationship between the rate of treatment failure among 25 symptomatic Plasmodium falciparum-infected patients presenting at the clinic and the pattern of resistance-associated SNPs in P. falciparum infecting 76 asymptomatic individuals from the surrounding population was investigated. The study was conducted in the SI in 2004. Patients presenting at a local clinic with microscopically confirmed P. falciparum malaria were recruited and treated with CQ+SP. Rates of treatment failure were estimated during a 28-day follow-up period. In parallel, a DNA microarray technology was used to analyse mutations associated with CQ, SP, and artemisinin derivative resistance among samples from the asymptomatic community. Mutation and haplotype frequencies were determined, as well as the multiplicity of infection.
Results
The in vivo study showed an efficacy of 88% for CQ+SP to treat P. falciparum infections. DNA microarray analyses indicated a low diversity in the parasite population with one major haplotype present in 98.7% of the cases. It was composed of fixed mutations at position 86 in pfmdr1, positions 72, 75, 76, 220, 326 and 356 in pfcrt, and positions 59 and 108 in pfdhfr. No mutation was observed in pfdhps or in pfATPase6. The mean multiplicity of infection was 1.39.
Conclusion
This work provides the first insight into drug resistance markers of P. falciparum in the SI. The obtained results indicated the presence of a very homogenous P. falciparum population circulating in the community. Although CQ+SP could still clear most infections, seven fixed mutations associated with CQ resistance and two fixed mutations related to SP resistance were observed. Whether the absence of mutations in pfATPase6 indicates the efficacy of artemisinin derivatives remains to be proven.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum.
- Record: found
- Abstract: found
- Article: not found
Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria.
- Record: found
- Abstract: found
- Article: not found