- Record: found
- Abstract: found
- Article: not found
Anti-PEG Antibodies Boosted in Humans by SARS-CoV-2 Lipid Nanoparticle mRNA Vaccine

Read this article at
Abstract
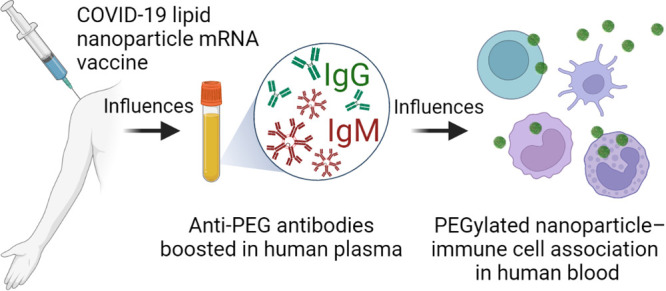
Humans commonly have low level antibodies to poly(ethylene) glycol (PEG) due to environmental exposure. Lipid nanoparticle (LNP) mRNA vaccines for SARS-CoV-2 contain small amounts of PEG, but it is not known whether PEG antibodies are enhanced by vaccination and what their impact is on particle–immune cell interactions in human blood. We studied plasma from 130 adults receiving either the BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) mRNA vaccines or no SARS-CoV-2 vaccine for PEG-specific antibodies. Anti-PEG IgG was commonly detected prior to vaccination and was significantly boosted a mean of 13.1-fold (range 1.0–70.9) following mRNA-1273 vaccination and a mean of 1.78-fold (range 0.68–16.6) following BNT162b2 vaccination. Anti-PEG IgM increased 68.5-fold (range 0.9–377.1) and 2.64-fold (0.76–12.84) following mRNA-1273 and BNT162b2 vaccination, respectively. The rise in PEG-specific antibodies following mRNA-1273 vaccination was associated with a significant increase in the association of clinically relevant PEGylated LNPs with blood phagocytes ex vivo. PEG antibodies did not impact the SARS-CoV-2 specific neutralizing antibody response to vaccination. However, the elevated levels of vaccine-induced anti-PEG antibodies correlated with increased systemic reactogenicity following two doses of vaccination. We conclude that PEG-specific antibodies can be boosted by LNP mRNA vaccination and that the rise in PEG-specific antibodies is associated with systemic reactogenicity and an increase of PEG particle–leukocyte association in human blood. The longer-term clinical impact of the increase in PEG-specific antibodies induced by lipid nanoparticle mRNA vaccines should be monitored. It may be useful to identify suitable alternatives to PEG for developing next-generation LNP vaccines to overcome PEG immunogenicity in the future.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection
- Record: found
- Abstract: found
- Article: not found
Doxil®--the first FDA-approved nano-drug: lessons learned.
- Record: found
- Abstract: found
- Article: not found
