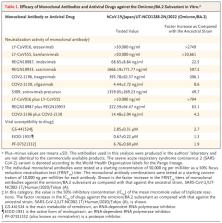
To the Editor: In November 2021, the B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was detected in South Africa. 1 Since then, omicron has rapidly spread around the world. On November 26, 2021, the World Health Organization designated omicron as a variant of concern. The omicron variant was found to have at least 33 mutations (29 amino acid substitutions, 1 insertion of three amino acids, and 3 small deletions) in its spike (S) protein, as compared with early SARS-CoV-2 strains identified in Wuhan, China. 2 Notably, 15 of the 29 substitutions were in the receptor-binding domain of the S protein, which is the primary target for monoclonal antibody–based therapy. This finding suggests that the monoclonal antibodies that have been approved by the Food and Drug Administration (FDA) may be less effective against the omicron variant. Accordingly, we examined the neutralizing ability of FDA-approved and investigational therapeutic monoclonal antibodies (individually and in combination) against omicron and other variants of concern. Using a live-virus focus reduction neutralization assay (FRNT), we assessed the neutralizing activities of monoclonal antibodies against hCoV-19/Japan/NC928-2N/2021 (omicron; NC928), which was isolated from a traveler who arrived in Japan from Namibia; SARS-CoV-2/UT-NC002-1T/Human/2020/Tokyo (NC002), an early SARS-CoV-2 strain from February 2020; SARS-CoV-2/UT-HP127-1Nf/Human/2021/Tokyo (alpha; HP127); hCoV-19/USA/MD-HP01542/2021 (beta; HP01542); hCoV-19/Japan/TY7-503/2021 (gamma; TY7-503); and hCoV-19/USA/WI-UW-5250/2021 (delta; UW5250). Whole-genome sequencing analysis of the NC928 omicron virus stock revealed that the variant had the 15 substitutions that are characteristic of omicron in the receptor-binding domain of the S protein, as compared with the Wuhan/Hu-1/2019 reference strain (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). We validated the reactivity of all seven monoclonal antibodies by means of enzyme-linked immunosorbent assay (ELISA) coated with recombinant S protein derived from the early Wuhan reference strain, as well as from representative alpha, beta, gamma, and delta variants. The results were consistent with published data 3 (Table S2). These monoclonal antibodies neutralized the early strain (NC002) and the alpha (HP127) and delta (UW5250) variants with a low FRNT50 value (1.34 to 150.38 ng per milliliter), except for LY-CoV555 (marketed as bamlanivimab), which showed markedly higher FRNT50 values against the delta variant than against the early strain and the alpha variant (Table 1). This result was consistent with a previous study that showed an almost complete loss of activity for bamlanivimab against the delta variant, whereas LY-CoV016 (marketed as etesevimab), REGN10987 (marketed as imdevimab), and REGN10933 (marketed as casirivimab) inhibited this variant. 4 Etesevimab did not neutralize the omicron (NC928), beta (HP01542), or gamma (TY7-503) variants even at the highest FRNT50 value (>50,000 ng per milliliter) that was tested. Bamlanivimab showed reduced neutralizing activity against the beta and gamma variants and did not neutralize omicron. Imdevimab had high neutralizing activity against the beta and gamma variants but lost activity against omicron. Casirivimab neutralized beta, gamma, and omicron with a high FRNT50 value (187.69 to 14,110.70 ng per milliliter); however, the FRNT50 value for omicron was higher by a factor of 18.6 than that for beta and higher by a factor of 75.2 than that for gamma. COV2-2196 (marketed as tixagevimab), COV2-2130 (marketed as cilgavimab), and S309 (precursor of drug marketed as sotrovimab) also retained neutralizing activity against beta, gamma, and omicron; however, the FRNT50 values of these monoclonal antibodies were higher by a factor of 3.7 to 198.2 for omicron than for beta or gamma. All the combinations of monoclonal antibodies that were tested (i.e., etesevimab plus bamlanivimab, imdevimab plus casirivimab, and tixagevimab plus cilgavimab) neutralized the early strain and the alpha and delta variants. The combination of etesevimab plus bamlanivimab showed remarkably reduced neutralizing activity against gamma and lost neutralizing activity against omicron and beta. The imdevimab–casirivimab combination retained activity against beta and gamma but lost inhibitory capability against omicron. The tixagevimab–cilgavimab combination inhibited beta, gamma, and omicron; however, the FRNT50 values of this combination were higher by a factor of 24.8 to 142.9 for omicron than for beta or gamma, respectively. The omicron variant has mutations in both the RNA-dependent RNA polymerase (RdRp) and the main protease of SARS-CoV-2, which are targets for antiviral drugs such as RdRp inhibitors (remdesivir and molnupiravir) and the main protease inhibitor PF-07304814, 5 which arouses concern regarding the decreased effectiveness of these drugs against omicron. Thus, we tested three different antiviral compounds (i.e., remdesivir, molnupiravir, and PF-07304814) for their efficacy against omicron. The in vitro 50% inhibitory concentration (IC50) values of each compound were determined against NC928, NC002, HP127, HP01542, TY7-503, and UW5250. The susceptibilities of omicron to the three compounds were similar to those of the early strain (i.e., IC50 values for remdesivir, molnupiravir, and PF-07304814 that differed by factors of 1.2, 0.8, and 0.7, respectively) (Table 1). These results suggest that all three of these compounds may show efficacy for treating patients infected with the omicron variant. The potential limitations of our study include the lack of clinical data on the efficacy of these monoclonal antibodies and antiviral drugs for the treatment of patients infected with omicron. Additional studies are needed to determine whether these antiviral therapies are indeed effective against infection with the omicron variant. Collectively, our findings show that therapeutic options may be available to combat the omicron variant of SARS-CoV-2; however, some therapeutic monoclonal antibodies may not be effective against this variant.