- Record: found
- Abstract: found
- Article: found
Intestinal Dysbiosis Associated with Systemic Lupus Erythematosus
Read this article at
ABSTRACT
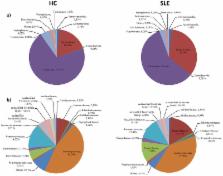
Systemic lupus erythematosus (SLE) is the prototypical systemic autoimmune disease in humans and is characterized by the presence of hyperactive immune cells and aberrant antibody responses to nuclear and cytoplasmic antigens, including characteristic anti–double-stranded DNA antibodies. We performed a cross-sectional study in order to determine if an SLE-associated gut dysbiosis exists in patients without active disease. A group of 20 SLE patients in remission, for which there was strict inclusion and exclusion criteria, was recruited, and we used an optimized Ion Torrent 16S rRNA gene-based analysis protocol to decipher the fecal microbial profiles of these patients and compare them with those of 20 age- and sex-matched healthy control subjects. We found diversity to be comparable based on Shannon’s index. However, we saw a significantly lower Firmicutes/ Bacteroidetes ratio in SLE individuals (median ratio, 1.97) than in healthy subjects (median ratio, 4.86; P < 0.002). A lower Firmicutes/ Bacteroidetes ratio in SLE individuals was corroborated by quantitative PCR analysis. Notably, a decrease of some Firmicutes families was also detected. This dysbiosis is reflected, based on in silico functional inference, in an overrepresentation of oxidative phosphorylation and glycan utilization pathways in SLE patient microbiota.
IMPORTANCE
Growing evidence suggests that the gut microbiota might impact symptoms and progression of some autoimmune diseases. However, how and why this microbial community influences SLE remains to be elucidated. This is the first report describing an SLE-associated intestinal dysbiosis, and it contributes to the understanding of the interplay between the intestinal microbiota and the host in autoimmune disorders.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: found
Diversity of Bifidobacteria within the Infant Gut Microbiota
- Record: found
- Abstract: found
- Article: not found