- Record: found
- Abstract: found
- Article: found
Neurochemical evidence supporting dopamine D1–D2 receptor heteromers in the striatum of the long-tailed macaque: changes following dopaminergic manipulation
Read this article at
Abstract
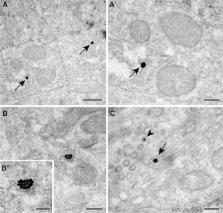
Although it has long been widely accepted that dopamine receptor types D1 and D2 form GPCR heteromers in the striatum, the presence of D1–D2 receptor heteromers has been recently challenged. In an attempt to properly characterize D1–D2 receptor heteromers, here we have used the in situ proximity ligation assay (PLA) in striatal sections comprising the caudate nucleus, the putamen and the core and shell territories of the nucleus accumbens. Experiments were carried out in control macaques as well as in MPTP-treated animals (with and without dyskinesia). Obtained data support the presence of D1–D2 receptor heteromers within all the striatal subdivisions, with the highest abundance in the accumbens shell. Dopamine depletion by MPTP resulted in an increase of D1–D2 density in caudate and putamen which was normalized by levodopa treatment. Two different sizes of heteromers were consistently found, thus suggesting that besides individual heteromers, D1–D2 receptor heteromers are sometimes organized in macromolecular complexes made of a number of D1–D2 heteromers. Furthermore, the PLA technique was combined with different neuronal markers to properly characterize the identities of striatal neurons expressing D1–D2 heteromers. We have found that striatal projection neurons giving rise to either the direct or the indirect basal ganglia pathways expressed D1–D2 heteromers. Interestingly, macromolecular complexes of D1–D2 heteromers were only found within cholinergic interneurons. In summary, here we provide overwhelming proof that D1 and D2 receptors form heteromeric complexes in the macaque striatum, thus representing a very appealing target for a number of brain diseases involving dopamine dysfunction.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay.
- Record: found
- Abstract: found
- Article: not found