- Record: found
- Abstract: found
- Article: found
Protein C-Terminal Tyrosine Conjugation via Recyclable Immobilized BmTYR
Read this article at
Abstract

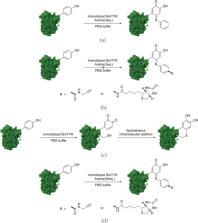
Protein modification plays an essential role in biological and pharmaceutical research. Due to the ordinary selectivity and inevitable damage to proteins of chemical synthetic methods, increased efforts were focused on biocatalysts which exhibited high regioselectivity and mild reaction conditions. However, separation of the biocatalysts and modified proteins remained a problem, especially when scaling up. Here, we developed a simple method for site-specific protein modification with a recyclable biocatalyst. The immobilizing tyrosinase (BmTYR) on magnetic beads can oxidize C-terminal tyrosine residues of the target protein to o-quinone, followed by the spontaneous addition of different nucleophiles (e.g., aniline derivatives), resulting in a C-terminal modified protein. Compared to the homogeneous biocatalytic system reported before, this heterogeneous system leads to an easier separation. Furthermore, the solid-phase biocatalyst can be regenerated during separation, providing reusability and lower costs.
Related collections
Most cited references41
- Record: found
- Abstract: not found
- Article: not found
Click Chemistry: Diverse Chemical Function from a Few Good Reactions
- Record: found
- Abstract: found
- Article: not found
Trypsin cleaves exclusively C-terminal to arginine and lysine residues.
- Record: found
- Abstract: found
- Article: not found