- Record: found
- Abstract: found
- Article: found
Advances in ultrasound-assisted synthesis of photocatalysts and sonophotocatalytic processes: A review
Read this article at
Summary
Water pollution and the global energy crisis are two significant challenges that the world is facing today. Ultrasound-assisted synthesis offers a simple, versatile, and green synthetic tool for nanostructured materials that are often unavailable by traditional synthesis. Furthermore, the integration of ultrasound and photocatalysis has recently received considerable interest due to its potential for environmental remediation as a low-cost, efficient, and environmentally friendly technique. The underlying principles and mechanisms of sonophotocatalysis, including enhanced mass transfer, improved catalyst-pollutant interaction, and reactive species production have been discussed. Various organic pollutants as dyes, pharmaceuticals, pesticides, and emerging organic pollutants are targeted based on their improved sonophotocatalytic degradation efficiency. Additionally, the important factors affecting sonophotocatalytic processes and the advantages and challenges associated with these processes are discussed. Overall, this review provides a comprehensive understanding of sono-assisted synthesis and photocatalytic degradation of organic pollutants and prospects for progress in this field.
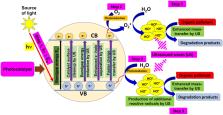
Graphical abstract
Abstract
Chemical reaction; Catalysis
Related collections
Most cited references140
- Record: found
- Abstract: found
- Article: not found
Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: a comparative overview
- Record: found
- Abstract: found
- Article: not found
