- Record: found
- Abstract: not found
- Article: not found
Mesenchymal stem cell-derived exosomes for clinical use
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: found
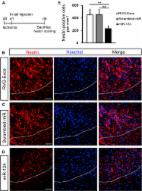
Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia
Jialei Yang, Xiufen Zhang, Xiangjie Chen … (2017)

- Record: found
- Abstract: found
- Article: found
Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases
Wael Nassar, Mervat El-Ansary, Dina Sabry … (2016)

- Record: found
- Abstract: found
- Article: found
Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents
Verena Börger, Michel Bremer, Rita Ferrer-Tur … (2017)
