- Record: found
- Abstract: found
- Article: found
Arginine Metabolism in Myeloid Cells Shapes Innate and Adaptive Immunity
Read this article at
Abstract
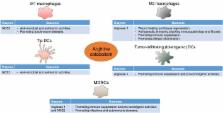
Arginine metabolism has been a key catabolic and anabolic process throughout the evolution of the immune response. Accruing evidence indicates that arginine-catabolizing enzymes, mainly nitric oxide synthases and arginases, are closely integrated with the control of immune response under physiological and pathological conditions. Myeloid cells are major players that exploit the regulators of arginine metabolism to mediate diverse, although often opposing, immunological and functional consequences. In this article, we focus on the importance of arginine catabolism by myeloid cells in regulating innate and adaptive immunity. Revisiting this matter could result in novel therapeutic approaches by which the immunoregulatory nodes instructed by arginine metabolism can be targeted.
Related collections
Most cited references114
- Record: found
- Abstract: found
- Article: not found
Nitric oxide and macrophage function.
- Record: found
- Abstract: found
- Article: not found
GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase.
- Record: found
- Abstract: found
- Article: not found