- Record: found
- Abstract: found
- Article: found
MRD dynamics during maintenance for improved prognostication of 1280 patients with myeloma in the TOURMALINE-MM3 and -MM4 trials

Read this article at
Key Points
Visual Abstract
Abstract
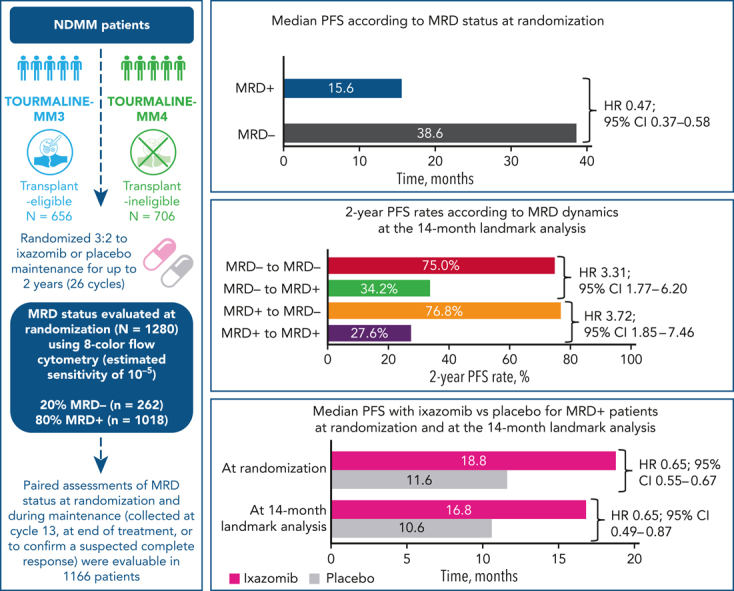
Measurable residual disease (MRD) evaluation may help to guide treatment duration in multiple myeloma (MM). Paradoxically, limited longitudinal data exist on MRD during maintenance. We investigated the prognostic value of MRD dynamics in 1280 transplant-eligible and -ineligible patients from the TOURMALINE-MM3 and -MM4 randomized placebo-controlled phase 3 studies of 2-year ixazomib maintenance. MRD status at randomization showed independent prognostic value (median progression-free survival [PFS], 38.6 vs 15.6 months in MRD − vs MRD + patients; HR, 0.47). However, MRD dynamics during maintenance provided more detailed risk stratification. A 14-month landmark analysis showed prolonged PFS in patients converting from MRD + to MRD − status vs those with persistent MRD + status (76.8% vs 27.6% 2-year PFS rates). Prolonged PFS was observed in patients with sustained MRD − status vs those converting from MRD − to MRD + status (75.0% vs 34.2% 2-year PFS rates). Similar results were observed at a 28-month landmark analysis. Ixazomib maintenance vs placebo improved PFS in patients who were MRD + at randomization (median, 18.8 vs 11.6 months; HR, 0.65) or at the 14-month landmark (median, 16.8 vs 10.6 months; HR, 0.65); no difference was observed in patients who were MRD −. This is the largest MM population undergoing yearly MRD evaluation during maintenance reported to date. We demonstrate the limited prognostic value of a single–time point MRD evaluation, because MRD dynamics over time substantially impact PFS risk. These findings support MRD − status as a relevant end point during maintenance and confirm the increased progression risk in patients converting to MRD + from MRD − status. These trials were registered at www.clinicaltrials.gov as #NCT02181413 and #NCT02312258.
Abstract
Measurable residual disease (MRD) status after initial therapy for multiple myeloma is a proven surrogate for progression-free survival (PFS), but its value during maintenance is uncertain. Paiva et al report that serial monitoring of MRD during maintenance treatment captures dynamic conversion between MRD-negative and -positive states and robustly anticipates PFS. This shows the feasibility of longer term MRD monitoring and provides a rationale for trials that investigate intervention prior to frank relapse or shortening of maintenance for those who remain MRD negative.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma.

- Record: found
- Abstract: found
- Article: found
Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma
- Record: found
- Abstract: found
- Article: not found
A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.