- Record: found
- Abstract: found
- Article: found
Lithium-Responsive Seizure-Like Hyperexcitability Is Caused by a Mutation in the Drosophila Voltage-Gated Sodium Channel Gene paralytic
Read this article at
Abstract
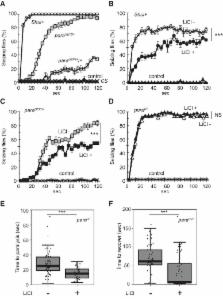
Shudderer ( Shu) is an X-linked dominant mutation in Drosophila melanogaster identified more than 40 years ago. A previous study showed that Shu caused spontaneous tremors and defects in reactive climbing behavior, and that these phenotypes were significantly suppressed when mutants were fed food containing lithium, a mood stabilizer used in the treatment of bipolar disorder ( Williamson, 1982). This unique observation suggested that the Shu mutation affects genes involved in lithium-responsive neurobiological processes. In the present study, we identified Shu as a novel mutant allele of the voltage-gated sodium (Na v) channel gene paralytic ( para). Given that hypomorphic para alleles and RNA interference–mediated para knockdown reduced the severity of Shu phenotypes, Shu was classified as a para hypermorphic allele. We also demonstrated that lithium could improve the behavioral abnormalities displayed by other Na v mutants, including a fly model of the human generalized epilepsy with febrile seizures plus. Our electrophysiological analysis of Shu showed that lithium treatment did not acutely suppress Na v channel activity, indicating that the rescue effect of lithium resulted from chronic physiological adjustments to this drug. Microarray analysis revealed that lithium significantly alters the expression of various genes in Shu, including those involved in innate immune responses, amino acid metabolism, and oxidation-reduction processes, raising the interesting possibility that lithium-induced modulation of these biological pathways may contribute to such adjustments. Overall, our findings demonstrate that Na v channel mutants in Drosophila are valuable genetic tools for elucidating the effects of lithium on the nervous system in the context of neurophysiology and behavior.
Related collections
Most cited references96
- Record: found
- Abstract: found
- Article: not found
Animal models of neuropsychiatric disorders.
- Record: found
- Abstract: found
- Article: not found
Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants.
- Record: found
- Abstract: found
- Article: not found