- Record: found
- Abstract: found
- Article: found
Applications of 2-deoxy-2-fluoro-D-glucose (FDG) in Plant Imaging: Past, Present, and Future
Read this article at
Abstract
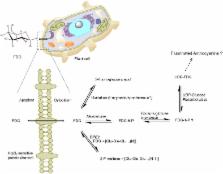
The aim of this review article is to explore and establish the current status of 2-deoxy-2-fluoro- D-glucose (FDG) applications in plant imaging. In the present article, we review the previous literature on its experimental merits to formulate a consistent and inclusive picture of FDG applications in plant-imaging research. 2-deoxy-2-fluoro- D-glucose is a [ 18F]fluorine-labeled glucose analog in which C-2 hydroxyl group has been replaced by a positron-emitting [ 18F] radioisotope. As FDG is a positron-emitting radiotracer, it could be used in in vivo imaging studies. FDG mimics glucose chemically and structurally. Its uptake and distribution are found to be similar to those of glucose in animal models. FDG is commonly used as a radiotracer for glucose in medical diagnostics and in vivo animal imaging studies but rarely in plant imaging. Tsuji et al. (2002) first reported FDG uptake and distribution in tomato plants. Later, Hattori et al. (2008) described FDG translocation in intact sorghum plants and suggested that it could be used as a tracer for photoassimilate translocation in plants. These findings raised interest among other plant scientists, which has resulted in a recent surge of articles involving the use of FDG as a tracer in plants. There have been seven studies describing FDG-imaging applications in plants. These studies describe FDG applications ranging from monitoring radiotracer translocation to analyzing solute transport, root uptake, photoassimilate tracing, carbon allocation, and glycoside biosynthesis. Fatangare et al. (2015) recently characterized FDG metabolism in plants; such knowledge is crucial to understanding and validating the application of FDG in plant imaging research. Recent FDG studies significantly advance our understanding of FDG translocation and metabolism in plants but also raise new questions. Here, we take a look at all the previous results to form a comprehensive picture of FDG translocation, metabolism, and applications in plants. In conclusion, we summarize current knowledge, discuss possible implications and limitations of previous studies, point to open questions in the field, and comment on the outlook for FDG applications in plant imaging.
Related collections
Most cited references72
- Record: found
- Abstract: found
- Article: not found
Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution.
- Record: found
- Abstract: found
- Article: not found
Combined MRI-PET dissects dynamic changes in plant structures and functions.
- Record: found
- Abstract: found
- Article: not found