- Record: found
- Abstract: found
- Article: found
Distinct Epithelial Cell Profiles in Normal Versus Induced-Congenital Diaphragmatic Hernia Fetal Lungs
Read this article at
Abstract
Background
Recent studies identified a great diversity of cell types in precise number and position to create the architectural features of the lung that ventilation and respiration at birth depend on. With damaged respiratory function at birth, congenital diaphragmatic hernia (CDH) is one of the more severe causes of fetal lung hypoplasia with unspecified cellular dynamics.
Objectives
To characterize the epithelial cell tissue in hypoplastic lungs, a careful analysis regarding pulmonary morphology and epithelial cell profile was conducted from pseudoglandular-to-saccular phases in normal versus nitrofen-induced CDH rat lungs.
Design
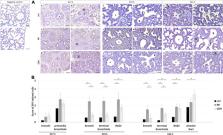
Our analysis comprises three experimental groups, control, nitrofen (NF) and CDH, in which the relative expression levels (western blot) by group and developmental stage were analyzed in whole lung. Spatiotemporal distribution (immunohistochemistry) was revealed by pulmonary structure during normal and hypoplastic fetal lung development. Surfactant protein-C (SP-C), calcitonin gene-related peptide (CGRP), clara cell secretory protein (CCSP), and forkhead box J1 (FOXJ1) were the used molecular markers for alveolar epithelial cell type 2 (AEC2), pulmonary neuroendocrine, clara, and ciliated cell profiles, respectively.
Results
Generally, we identified an aberrant expression of SP-C, CGRP, CCSP, and FOXJ1 in nitrofen-exposed lungs. For instance, the overexpression of FOXJ1 and CGRP in primordia of bronchiole defined the pseudoglandular stage in CDH lungs, whereas the increased expression of CGRP in bronchi; FOXJ1 and CGRP in terminal bronchiole; and SP-C in BADJ classified the canalicular and saccular stages in hypoplastic lungs. We also described higher expression levels in NF than CDH or control groups for both FOXJ1 in bronchi, terminal bronchiole and BADJ at canalicular stage, and SP-C in bronchi and terminal bronchiole at canalicular and saccular stages. Finally, we report an unexpected expression of FOXJ1 in BADJ at canalicular and saccular stages, whereas the multi cilia observed in bronchi were notably absent at embryonic day 21.5 in induced-CDH lungs.
Related collections
Most cited references65
- Record: found
- Abstract: found
- Article: not found
Alveolar progenitor and stem cells in lung development, renewal and cancer.
- Record: found
- Abstract: found
- Article: not found