- Record: found
- Abstract: found
- Article: found
全反式维甲酸对NPM1突变的白血病细胞系U937细胞的作用及其机制研究 Translated title: Study of the effects and mechanism of all-trans retinoic acid on leukemic cell line U937 cells with NPM1 mutation
Read this article at
Abstract
方法
转染NPM1突变型(A型)质粒至U937细胞系构建稳定克隆A1和A2,利用Western blot法和荧光共聚焦技术鉴定细胞。MTT法检测细胞增殖活性,流式细胞术检测细胞周期和细胞凋亡,显微镜下计数检测集落形成能力,Western blot法检测细胞增殖相关信号通路蛋白表达。
结果
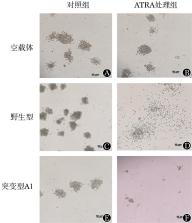
①NPM1突变型U937细胞A1和A2经ATRA处理后,细胞增殖活性分别为对照组(未经ATRA处理组)水平的52.6%和35.8%,差异均有统计学意义( t=3.649, P=0.010; t=4.906, P=0.002)。②突变型细胞A1和A2的G 0/G 1期比例经ATRA处理后较对照组分别增加20.1%和35.8%,差异均有统计学意义( t=4.544, P=0.010; t=15.850, P<0.001)。③U937细胞经ATRA处理后集落生长受抑制,其中空载体组集落数目下降了32.7%,野生型组下降了57.9%,突变型组(A1)下降了90.9%,差异均有统计学意义( t=8.507, P=0.010; t=22.090, P<0.001; t=90.200, P<0.001)。④突变型U937细胞经ATRA处理后p-ERK水平明显降低。⑤突变型U937细胞接受化疗药物联合ATRA处理后细胞凋亡率明显高于化疗药物单药处理组,在化疗药物处理后添加ATRA能导致更多的细胞凋亡。
Translated abstract
To investigate the effect and mechanism of all-trans retinoic acid (ATRA) on leukemic cell line U937 cells with NPM1 mutation.
Human acute myeloid leukemia cell line U937 was explored, NPM1 mutated (A type) plasmids were transfected into U937 to form stable clones A1 and A2, which were identified by Western blot and Co-immunoprecipitation. The cell proliferation was measured by methylthiazolyl tetrazolium bromide (MTT); cell cycle and cell apoptosis were explored by flow cytometric; cell colony formation was measured by microscope count, the molecular pathways related to cell proliferation were measured by Western blot.
①The cell proliferations of mutant A1 and A2 were inhibited significantly by 52.6% and 35.8% ( P<0.05), respectively under ATRA exposure. ②The percentages of G 0/G 1 stage of mutant A1 and A2 increased by 20.1% and 35.8%, respectively under ATRA exposure. ③All the U937 leukemic cells were inhibited under ATRA exposure; the decreased percentages of vector, wild-type and mutant NPM1 cells were 32.7%, 57.9% and 90.9% respectively. ④p-ERK decreased obviously after ATRA exposure in NPM1 mutated leukemic cells. ⑤More mutant NPM1 cells inclined to apoptosis under the exposure of ATRA and cytotoxic drugs than cytotoxic drugs alone, meanwhile more cells apoptosis occurred when ATRA was administrated after cytotoxic drugs exposure.
ATRA could inhibit cell proliferation and colony formation, blocked the cell cycle in the G 0/G 1 stage accompanied by the significant reduction of p-ERK in U937 leukemic cells with NPM1 mutation. Besides, ATRA could synergize with drugs to suppress the leukemic cells survival more effectively when ATRA was administered after the cytotoxic drugs exposure in U937 leukemic cells with NPM1 mutation.
Related collections
Most cited references16
- Record: found
- Abstract: found
- Article: not found
Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia.
- Record: found
- Abstract: found
- Article: not found
Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet.
- Record: found
- Abstract: found
- Article: not found