- Record: found
- Abstract: found
- Article: found
Faecal immunochemical testing (FIT) in patients with signs or symptoms of suspected colorectal cancer (CRC): a joint guideline from the Association of Coloproctology of Great Britain and Ireland (ACPGBI) and the British Society of Gastroenterology (BSG)
Read this article at
Abstract
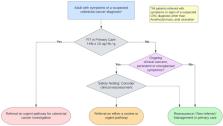
Faecal immunochemical testing (FIT) has a high sensitivity for the detection of colorectal cancer (CRC). In a symptomatic population FIT may identify those patients who require colorectal investigation with the highest priority. FIT offers considerable advantages over the use of symptoms alone, as an objective measure of risk with a vastly superior positive predictive value for CRC, while conversely identifying a truly low risk cohort of patients. The aim of this guideline was to provide a clear strategy for the use of FIT in the diagnostic pathway of people with signs or symptoms of a suspected diagnosis of CRC. The guideline was jointly developed by the Association of Coloproctology of Great Britain and Ireland/British Society of Gastroenterology, specifically by a 21-member multidisciplinary guideline development group (GDG). A systematic review of 13 535 publications was undertaken to develop 23 evidence and expert opinion-based recommendations for the triage of people with symptoms of a suspected CRC diagnosis in primary care. In order to achieve consensus among a broad group of key stakeholders, we completed an extended Delphi of the GDG, and also 61 other individuals across the UK and Ireland, including by members of the public, charities and primary and secondary care. Seventeen research recommendations were also prioritised to inform clinical management.
Related collections
Most cited references143
- Record: found
- Abstract: found
- Article: not found
Colorectal cancer statistics, 2017.
- Record: found
- Abstract: not found
- Article: not found
What is "quality of evidence" and why is it important to clinicians?

- Record: found
- Abstract: found
- Article: found