- Record: found
- Abstract: found
- Article: found
A systematic review of the clinical presentation, treatment and relapse characteristics of human Plasmodium ovale malaria
Read this article at
Abstract
Background
Despite increased efforts to control and ultimately eradicate human malaria, Plasmodium ovale malaria is for the most part outside the focus of research or public health programmes. Importantly, the understanding of P. ovale—nowadays regarded as the two distinct species P. ovale wallikeri and P. ovale curtisi—largely stems from case reports and case series lacking study designs providing high quality evidence. Consecutively, there is a lack of systematic evaluation of the clinical presentation, appropriate treatment and relapse characteristics of P. ovale malaria. The aim of this systematic review is to provide a systematic appraisal of the current evidence for severe manifestations, relapse characteristics and treatment options for human P. ovale malaria.
Methods and results
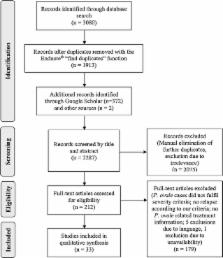
This systematic review was performed according to the PRISMA guidelines and registered in the international prospective register for systematic reviews (PROSPERO 2016:CRD42016039214). P. ovale mono-infection was a strict inclusion criterion. Of 3454 articles identified by the literature search, 33 articles published between 1922 and 2015 met the inclusion criteria. These articles did not include randomized controlled trials. Five prospective uncontrolled clinical trials were performed on a total of 58 participants. P. ovale was sensitive to all tested drugs within the follow-up periods and on interpretable in vitro assays. Since its first description in 1922, only 18 relapsing cases of P. ovale with a total of 28 relapse events were identified in the scientific literature. There was however no molecular evidence for a causal relationship between dormant liver stages and subsequent relapses. A total of 22 severe cases of P. ovale malaria were published out of which five were fatal. Additionally, two cases of congenital P. ovale malaria were reported.
Conclusions
Current knowledge of P. ovale malaria is based on small trials with minor impact, case reports and clinical observations. This systematic review highlights that P. ovale is capable of causing severe disease, severe congenital malaria and may even lead to death. Evidence for relapses in patients with P. ovale malaria adds up to only a handful of cases. Nearly 100 years after P. ovale’s first description by Stephens the evidence for the clinical characteristics, relapse potential and optimal treatments for P. ovale malaria is still scarce.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally.

- Record: found
- Abstract: found
- Article: not found