- Record: found
- Abstract: not found
- Article: not found
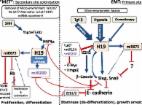
lncRNA H19 prevents endothelial–mesenchymal transition in diabetic retinopathy
January 5 2019
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases.
Thomas Wynn (2007)
- Record: found
- Abstract: found
- Article: found
The H19 Long non-coding RNA in cancer initiation, progression and metastasis – a proposed unifying theory
Eli Raveh, Imad Matouk, Michal Gilon … (2015)
- Record: found
- Abstract: found
- Article: not found
The H19 locus: role of an imprinted non-coding RNA in growth and development.
Anne Gabory, Hélène Jammes, Luisa Dandolo (2010)
