Introduction
The 2019 novel coronavirus disease (COVID-19), caused by the SARS-CoV-2 virus, has widely spread throughout the world. The number of infected people continues to increase, thus resulting in serious concerns regarding the public health and economic development of all countries [1, 2].
Beyond affecting the respiratory system, COVID-19 can also damage multiple organs, such as the heart, liver, brain, kidneys, and spleen (Figure 1). Myocardial injury and myocarditis, important complications of COVID-19, have attracted widespread attention because they are closely associated with patient prognosis. Data are increasingly indicating that a substantial proportion of patients with COVID-19 also develop cardiovascular complications (Table 1).
Myocardial Involvement in Patients with COVID-19.
| Patients with COVID-19 | Cardiovascular complications | Location of cases | Publication year |
|---|---|---|---|
| 41 hospitalized patients [3] | 12.5% virus-related heart damage | Wuhan, China | 2020 |
| 148 hospitalized patients [4] | 26% myocarditis scar, 22% infarction and/or ischemia, 6% dual pathology | Wuhan, China | 2021 |
| 150 hospitalized patients [5] | 40% of deaths attributed to cardiac disease | Wuhan, China | 2020 |
| 187 hospitalized patients [6] | 27.8% heart damage | Wuhan, China | 2020 |
| 278 hospitalized patients [7] | 23.7% cardiovascular complications | Wuhan, China | 2020 |
| 416 hospitalized patients [8] | 19.7% heart damage | Wuhan, China | 2020 |
| 1527 hospitalized patients [9] | 16.4% cardiovascular and cerebrovascular diseases | China | 2020 |
| 100 recovered patients [10] | 78% and 60% cardiac involvement and persistent myocardial inflammation | Germany | 2020 |
| 189 hospitalized patients [11] | 16% myocardial injury | Rome, Italy | 2021 |
| 313 hospitalized patients [12] | 27.2% myocardial injury | Italy | 2021 |
| 12577 hospitalized patients [13] | 9.2–51% myocardial injury | USA | 2021 |
| 4695 hospitalized patients [14] | 6.8% chronic myocardial injury, 24.9% acute myocardial injury | New York, USA | 2021 |
| 56963 hospitalized patients [15] | 97 patients with possible acute myocarditis | USA and Europe | 2022 |
| 153760 individuals with COVID-19 [16] | Substantial risk and 1-year burden of cardiovascular disease in survivors of acute COVID-19 | US Department of Veterans Affairs | 2022 |
In addition, a prior review has summarized more relevant information [17].
Herein, our aim was to review the occurrence and development of myocardial injury and myocarditis after COVID-19; to explore possible underlying mechanisms; and to review the available diagnostic tests for myocardial injury and myocarditis, and pharmacotherapy approaches for COVID-19 related cardiovascular complications.
We used the keywords “COVID-19,” “coronavirus,” “SARS-CoV-2,” “health consequence,” “cardiovascular,” “myocarditis,” “myocardial injury,” and “cardiac injury” to search the Medline and PubMed databases. The search was not limited to English articles and was unrestricted by timeframe. The last search was performed on April 8, 2023.
Relationship Between COVID-19 and Myocardial Involvement
Substantial evidence has demonstrated the existence of myocardial injury after COVID-19 [18]. Cardiac dysfunction worsens with the progression of COVID-19 [19]. Moreover, heart damage itself may worsen the condition of patients with COVID-19. Patients with COVID-19 with myocardial injury have a higher mortality rate than those without myocardial injury [8]. According to an investigation in 1527 patients with COVID-19, the incidence of acute cardiac injury in ICU/critically ill patients is 13 times higher than that in non-ICU/critically ill patients [9].
Furthermore, myocardial injury is more pronounced in patients with COVID-19 with than without concomitant medical diseases. Previous studies have indicated that older age, comorbidities (e.g., hypertension, coronary syndrome, chronic renal failure, and chronic obstructive pulmonary disease), and high levels of C-reactive protein (CRP) are predictors and risk factors of myocardial injury in patients with COVID-19 [19, 20]. In one study, analysis of cardiac biomarkers, such as creatine kinase, myoglobin, hypersensitivity troponin I, and b-type natriuretic peptide, in 49 patients with COVID-19 who received maintenance hemodialysis has indicated more severe cardiac damage in these patients than controls [21].
Myocardial Injury During COVID-19 Convalescence
A meta-analysis has reported that 63.2%, 71.9%, and 45.9% of infected patients exhibit at least one post-COVID-19 symptom at 30, 60, or ≥90 days after the onset of illness or hospitalization. Fatigue and dyspnea are the most prevalent symptoms, along with other symptoms such as headache, loss of the sense of smell, chest pain, and palpitations [22].
In an earlier U.S. interview of outpatients with mild COVID-19, 35% of the symptomatic respondents reported not having returned to their usual health state by the date of the interview (median=16 days from testing) [23].
A cohort study in 153,760 individuals with COVID-19 has estimated the risks and 1-year burdens of a set of pre-specified incident cardiovascular outcomes. Beyond the first 30 days after infection, individuals with COVID-19 are at elevated risk of several types of incident cardiovascular disease [16].
Moreover, cardiovascular magnetic resonance (CMR) has been used to visualize cardiac involvement in recently recovered patients with COVID-19 in a German study, in which the most prevalent abnormality was myocardial inflammation [10].
In another study, in 148 patients with severe COVID-19 and troponin elevation who underwent convalescent CMR at a median time of 68 days, myocarditis-like injury was frequently encountered, and some patients with COVID-19 showed potential ongoing localized inflammation. In addition, one-quarter of patients with COVID-19 developed ischemic heart disease manifestations [4].
Advanced cardiovascular complications may occur even in mild COVID-19. Hospitalized patients have increased risks of developing many conditions, including an 8% increase in the frequency of heart attacks and a 247% increase in the incidence of heart inflammation [24].
These findings indicate that further investigation of the long-term cardiovascular consequences of COVID-19 should be performed, particularly for cases with severe disease or clinical indications of cardiac damage.
Myocarditis During COVID-19 Convalescence
Among cardiovascular complications, the occurrence of explosive myocarditis has attracted attention. Recently, the Omicron variant has become very common, and preliminary reports have suggested that it leads to less severe disease than previous variants. However, Omicron may cause myocarditis [25]. Acute myocarditis is considered a rare cardiovascular complication of COVID-19 [15].
Myocarditis occurs in approximately 0.2 of every 1000 COVID-19 survivors. After recovering from COVID-19, people have a significantly higher risk of developing myocarditis within 1 year after the onset of infection [26].
Mortality in patients with fulminant myocarditis markedly increases with the occurrence of cardiogenic shock, ventricular arrhythmias, and multi-organ dysfunction. Arrhythmias are common in patients with COVID-19 with myocarditis [27].
Notably, many observations have indicated that COVID-19 mRNA vaccination is associated with an elevated risk of short-term myocarditis [28]. However, the exact mechanism remains unknown.
Patients presenting with chest pain, shortness of breath, syncope, edema, or other symptoms should undergo tests including ECG, UCG, and CMR. In this population, follow-up testing is recommended 3–6 months after onset of cardiovascular symptoms. Additionally, COVID-19 survivors, even those with mild cases, have been recommended to resume exercise no sooner than 7 days after symptoms have disappeared, and to perform a minimum amount of exercise in the first 2 weeks; monitor any uncomfortable symptoms; and seek medical attention as soon as possible even in the absence of symptoms of myocardial involvement, because the possibility of myocardial damage after recovery from COVID-19 is not negligible [29].
More data are necessary to enable more comprehensive conclusions to be drawn regarding possible future variants of SARS-CoV-2.
Pathogenesis of Myocardial Injury and Myocarditis in COVID-19
Myocardial injury and myocarditis are important pathogenic features of COVID-19. Virus-induced myocardial injury includes primary myocardial injury (direct invasion of myocardial cells by the virus or attack by inflammatory factors of the myocardium) and non-primary myocardial injury (multi-organ failure, hypoxia, hypoperfusion, and coagulopathy). The exact mechanisms of how SARS-CoV-2 causes myocardial injury and myocarditis have not been fully elucidated. The Angiotensin Converting Enzyme-2 (ACE2) receptor appears to play a critical role in SARS-CoV-2 pathogenesis. The ACE2 receptor has been demonstrated to limit the harmful effects of angiotensin II binding AT1 receptors, including vasoconstriction, increased inflammation, and thrombosis [30]. SARS-CoV-2 enters cells through membrane fusion, thus causing ACE2 to be down-regulated and subsequently lose its catalytic ability to degrade angiotensin II [30, 31]. Through single-cell RNA sequencing, the target organs for SARS-CoV-2 have been identified as the lungs, heart, esophagus, kidneys, bladder, and ileum; moreover, localized specific cell types (i.e., type II alveolar cells, myocardial cells, renal proximal tubule cells, ileal and esophageal epithelial cells, and bladder urothelial cells) have been found to be susceptible to COVID-19 [32].
Several key mechanisms of COVID-19 related myocardial injury are reviewed below.
Direct Viral Toxicity or Immunopathological Consequences
Direct viral toxicity or immunopathological consequences of COVID-19, including cytokine storms, lead to multi-organ damage, and cardiac injury is common [33]. With further elucidation of SARS-CoV-2 virology, the observed viral interaction with ACE2 in cardiomyocytes (CMs) supports a physiological rationale for direct viral damage to these cells [34, 35].
ACE2 is highly expressed in adult pericardial cells, thus suggesting an intrinsic cardiac susceptibility to SARS-CoV-2 infection. Pericyte damage caused by viral infection can lead to capillary endothelial cell dysfunction and subsequent microvascular dysfunction. Notably, people with underlying heart failure have elevated ACE2 expression at both the mRNA and protein levels, and consequently may be at increased risk of heart damage and critical illness when they are infected with SARS-CoV-2 [36].
On the basis of recent studies, endosomal dependence can compensate for S protein priming, thereby mediating SARS-CoV-2 infection of CMs. Findings of sarcomere disruption, myofibril loss, and altered gene expression profiles suggest that SARS-CoV-2 causes direct myocardial injury, which is promoted by IL-2 [35].
Furthermore, high CD47 levels contribute to vascular disease, vasoconstriction, and hypertension – conditions that may predispose SARS-CoV-2-infected individuals to COVID-19-associated complications (e.g., pulmonary hypertension, lung fibrosis, myocardial injury, stroke, and acute kidney injury). CD47 interferes with the host immune response through mechanisms including binding SIRPα on immune cells. In addition to enhancing expression of CD47 in different cell types, SARS-CoV-2 increases SIRPα levels in primary human monocytes [37]. These findings have revealed the potential role of the CD47/SIRPα axis in COVID-19 pathogenesis.
Moreover, proteolytic release of soluble ACE2 by A disintegrin and metalloproteinase (ADAM) 17 into the circulation decreases ACE2-mediated protection against the tissue renin-angiotensin-aldosterone system, thus contributing to multi-organ injury and severe extra pulmonary manifestations secondary to SARS-CoV-2–mediated pathology [38].
The Critical Role of the Inflammatory Response
Although direct viral toxicity and immunopathological consequences have been widely accepted, no current evidence indicates that myocarditis is a direct result of SARS-CoV-2 infection in humans. However, the lymphocytic myocarditis observed in patients with COVID-19 has been associated with the generalized inflammatory reaction induced by cytokines [39].
Leng et al. have established a region-resolved proteome map of the inflammatory myocardia and microvessels in the heart in patients with COVID-19, and have found that these structures are affected by an inflammation storm [40]. Virus-activated “cytokine storm syndrome” or fulminant myocarditis might be an important cause of patient death [5]. Multiple clinical studies have found a close correlation between myocardial injury and levels of inflammation, as indicated by various circulatory inflammation biomarkers such as CRP and IL-6 [41, 42]. Furthermore, patients with rather than without cardiac injury have higher proportions of high-sensitivity CRP, tumor necrosis factor (TNF)-α, interleukin-2 receptor (IL-2R), IL-6, and IL-8 [43].
T-cells and antigen-presenting cells are hyperactivated through Toll-like receptors after repeated stimulation, thus causing a massive autocrine feedback loop involving IL-1. Subsequently, pro-inflammatory cytokines including IL-6, IL-18, interferon (IFN), and TNFα; ferritin; and more clinical precipitating factors undergo uncontrolled release, thus leading to endothelial dysfunction, multiple organ dysfunction, and death [44].
Immunohistochemical analysis of the hearts of patients with COVID-19 has revealed elevated expression of caspase-1, ICAM-1, IL-1b, IL-6, MMP-9, TNF, and other markers. In one study, the histology of all analyzed COVID-19 myocardium samples has revealed severe pericardiocyte interstitial edema and elevated mast cell counts per high-power field. Increased TGF and interstitial collagen expression in COVID-19-affected hearts may potentially lead to chronic myocardial fibrosis [45]. Nevertheless, the myocardial damage in patients with COVID-19 is not believed to be classical lymphocytic myocarditis but a macrophage-dominated inflammatory pattern [46].
Both human pluripotent stem cell-derived CMs and adult CMs can be infected by SARS-CoV-2, thus leading to the secretion of the monocyte chemoattractant cytokine C-C motif chemokine ligand 2 (CCL2) and subsequent monocyte recruitment [47]. These findings may provide a theoretical basis for clinical immunotherapy in select patients with COVID-19, and might also serve as potential therapeutic targets for developing new treatments.
Effects of Oxidative Stress
As discussed in the previous section, SARS-CoV-2 infection causes ACE2 down-regulation, thus decreasing degradation of angiotensin II. Accumulated angiotensin II then activates the PI3K-Akt signaling pathway, and regulates endothelial activation and the production of reactive oxygen species (ROS) as well as IL-6 [48].
Studies have demonstrated that Nox2 is upregulated by RNA viruses, and induces myocardial injury and artery dysfunction via the production of ROS. Nox2 increases during the acute phase of community-acquired pneumonia, and is independently associated with troponin elevation and heart failure [49]. Similarly, patients with COVID-19 have been found to have Nox2 activation. Soluble Nox2-derived peptide (sNox2-dp), an indicator of Nox-2 activation, is higher in patients with COVID-19 than controls, and in patients with severe rather than non-severe COVID-19. Moreover, patients who experience thrombotic events have higher sNox-2-dp levels than those who do not [50]. These findings suggest that decreasing Nox2-associated ROS production might be a novel cardio-protective strategy in COVID-19 treatment.
Lack of Cardiac Reserve Capacity
The heart’s function as a blood-pumping organ, anti-virus defense, and adaptation to stress-induced viral infection in the body are all associated with the heart’s initial state. If underlying cardiovascular diseases coexist, inadequate cardiac reserve predisposes the heart to acute injury from viral infection. In the setting of COVID-19, tachycardia, hypotension, and hypoxemia can result in acute changes in myocardial demand and supply, thus contributing to acute myocardial injury, such as type 2 myocardial infarction, which refers to myocardial necrosis caused by an imbalance in myocardial oxygen supply and demand, in the absence of acute coronary thrombosis [51]. For example, hypoxemia induces vasoconstriction, increased myocardial demand, and ultimately myocardial injury due to supply demand mismatch [52]. Moreover, cardiovascular risk factors and chronic cardiovascular conditions are prevalent among patients affected by COVID-19 and are associated with adverse outcomes. Therefore, cardiac injury might potentially be due to the unmasking of underlying cardiovascular disease [53]. Overall, cardiovascular complications of COVID-19 should be an important concern, particularly in patients presenting with serious underlying cardiovascular conditions.
Micro-Thrombus, a Major Risk Factor for Myocardial Injury
A salient clinical feature of COVID-19 is the frequent incidence of microvascular thrombosis. COVID-19 autopsy reports have shown widespread thrombotic microangiopathy, characterized by extensively diffuse micro-thrombi within the peripheral capillaries and arterioles of the lungs, heart, and other organs, thus leading to multi-organ failure [54–59]. In addition, micro-thrombus formation plays a key role in myocardial injury. The most common etiology of myocyte necrosis is micro-thrombus formation [58].
Endothelial damage caused by SARS-CoV-2 and subsequent host response disorders involving inflammation and clotting pathways play a key role in the progression of severe COVID-19 [60]. To further reveal the underlying process of COVID-19-associated micro-thrombosis, researchers have analyzed the landscape of circulating platelet aggregates in COVID-19, through massive single-cell image-based profiling and temporal monitoring of the blood from 110 patients. Nearly 90% of patients with COVID-19 showed anomalous presence of excessive platelet aggregates, and the concentrations of platelet aggregates were strongly associated with the severity of the COVID-19 condition, mortality, respiratory condition, and vascular endothelial dysfunction level [61].
Drug-Induced Myocardial Injury
In the early stages of the COVID-19 pandemic, chloroquine and its analogue hydroxyl-chloroquine were used as an investigative treatment for SARS-CoV-2 infection. However, subsequent studies did not demonstrate their clinical benefit. Both drugs are known to have potential cardiotoxic adverse effects. They have been shown to be associated with significant QTc prolongation, as well as reports of ventricular arrhythmias in patients with COVID-19 [62]. Moreover, chloroquine and hydroxychloroquine are metabolized by CYP3A4. In addition to arrhythmic risks, they also cause direct myocardial injury. Patients treated with a combination of hydroxychloroquine and azithromycin have been observed to have a higher risk of experiencing cardiac arrest when compared to those treated with hydroxychloroquine or azithromycin alone [63].
Human induced pluripotent stem cell-derived CMs have been used to verify the toxicity of a batch of experimentally used drugs. Remdesivir, favipiravir, camostat, and ivermectin have shown little effect on field potentials, whereas hydroxychloroquine decreases the electro-mechanical window of human induced pluripotent stem cell-derived CMs in a concentration-dependent manner [64].
Furthermore, azithromycin, a macrolide antibiotic commonly used when a bacterial co-infection is suspected in COVID-19 cases, is also well known to cause QT prolongation [65]. Practitioners should therefore be cautious of these potential cardiotoxic agents when treating patients with COVID-19, who are prone to myocardial injury and arrhythmia.
Complex Systemic Response
Myocardial injury has been suggested to be associated with systemic consequences of SARS-CoV-2 infection rather than direct viral attack or myocarditis. In an autopsy cohort of 40 deceased patients with COVID-19, the SARS-CoV-2 genome was not identified in the CMs of patients with myocarditis, but was focally but negligibly present in CMs of patients with viral persistence in the lungs who presented no signs of myocardial inflammation. The presence of myocardial injury was not associated with myocardial inflammatory infiltrates [66]. An autopsy from another case report has also found no substantial histological changes in the cardiac tissue except for several interstitial mononuclear inflammatory infiltrates [67].
Clinical evidence from an earlier observational study has suggested that the elevation in cardiac markers is probably due to secondary and systemic consequences [20].
A study in 671 patients with severe COVID-19 has found that myocardial injury is significantly associated with cardiovascular comorbidities and the inflammatory response, as indicated by high levels of CRP [68]. The presence of comorbidities may be the most critical risk factor for cardiac damage in patients with COVID-19 [19], and the presence of comorbidities has been suggested to downregulate the immune system and predispose people to more severe viral invasion [68]. Meanwhile, patients with COVID-19 with normal versus elevated troponin levels have been found to differ in multiple indicators of organ function (i.e., heart, liver, kidneys, and lungs) [6].
Diagnostic Assessment of Myocardial Injury
Laboratory Tests
Troponin I elevation after COVID-19 is widely accepted to indicate myocardial injury rather than MI. The release of cTn from injured CMs through extracellular vesicle secretion into the blood may be a possible explanation for the cTn increase in COVID-19 [69–71]. In addition, a meta-analysis has indicated that acute cardiac injury, represented by elevated troponin concentrations, is strongly associated with high mortality, ICU care needs, and severity of COVID-19 [8, 18, 19].
In COVID-19, cTn can reasonably be used as a clinical tool to for triage of patients according to risk and to identify patients at high risk of cardiac impairment during both early and recovery stages of COVID-19 [11].
In addition to cTn, the concentrations of CK-MB, MYO, the-cTnI, and NT-proBNP are associated with COVID-19 severity and fatality [72, 73].
In patients with COVID-19, NT-proBNP, inflammatory markers such as D-dimer, ferritin, and CRP are elevated, thus suggesting multi-organ failure and a multisystem inflammatory response. However, even if the levels of other markers are normal, only elevated serum troponin levels indicate myocarditis [74].
Nevertheless, a combination of inflammation (i.e., CRP) and coagulation markers (D-dimer) may provide additional prognostic information in COVID-19 [75].
Some emerging markers of myocardial injury can predict COVID-19 severity and prognosis. ADAMTS13, the von Willebrand Factor-cleaving protease whose loss of function causes microvascular thrombosis, and FSTL3, an indicator of senescence-promoting Activin/TGFβ signaling, are among the proteins most strongly associated with myocardial stress and injury. Lower genetically determined levels of plasma ADAMTS13 have been found to be associated with a higher likelihood of clinically diagnosed myocardial injury in a large, general population. FSTL3 levels are strongly correlated with both NT-proBNP and cardiac troponin T, and have been found to be markedly higher in both moderate and severe COVID-19 cases with cardiac involvement than in controls [76]. Furthermore, elevated soluble Nox2-derived peptide (sNox2-dp) is found in severe COVID-19 cases and is associated with thrombotic events [50].
The Diagnostic Role of Imaging Examination
Cardiac imaging is widely used in the context of COVID-19 to detect myocardial damage or dysfunction. Cardiac imaging abnormalities together with elevated troponin have additional prognostic value. Echocardiography, because it is widely accessible and can be performed at the bedside, may be used as a first-level approach to enable timely diagnosis and monitoring of cardiac structure and function changes [77]. However, the accuracy of echocardiography may be limited by its relatively low spatial resolution and poor echo window, such as in patients with chronic obstructive pulmonary disease. More advanced imaging techniques such as CMR can serve as powerful backup tools in some cases. CMR is the gold standard noninvasive imaging test for the assessment of cardiac chamber size and function. Most importantly, it provides accurate myocardial tissue characterization with combined MR sequences (e.g., T2-weighted imaging, late enhancement sequence, and T1/T2 mapping) to visualize myocardial edema, necrosis, and fibrosis, thus aiding in clarifying myocardial injury patterns, such as myocarditis-like abnormalities or myocardial infarction-like necrosis [4, 78, 79].
Computed tomography is widely used for screening, diagnosis, and follow-up for patients with COVID-19 pneumonia. In addition, cardiac computed tomography can be used to assess and differentiate the etiology of myocardial injury. In patients with COVID-19 with elevated troponin, when acute coronary syndrome is suspected with low or intermediate probability, coronary angiography can be performed; if obstructive coronary disease is excluded, an additional delayed acquisition might be obtained for the detection of potential myocardial damage [80].
Other Inspection Methods
Myocardial injury, defined by elevated cardiac enzymes, is associated with abnormal ECG changes and myocardial dysfunction on echocardiography [81]. Patients with myocarditis may have a variety of arrhythmias, such as sinus tachycardia, ST-segment elevation, new-onset bundle branch block, PR depression, and QT prolongation [54, 76].
Combined with CMR, quality of life questionnaires, 6-minute walk tests, and 12-lead ECG enable comprehensive assessment of patient cardiac reserve [82]. Wide QRS complex and lateral ST-T segment abnormality are associated with poor clinical outcomes among patients with COVID-19 [76]. Furthermore, endomyocardial biopsy is another method of choice to assess myocardial injury in some cases, although it is invasive [69].
In summary, the diagnosis of myocardial injury in patients with COVID-19 has been comprehensive (Figure 2).
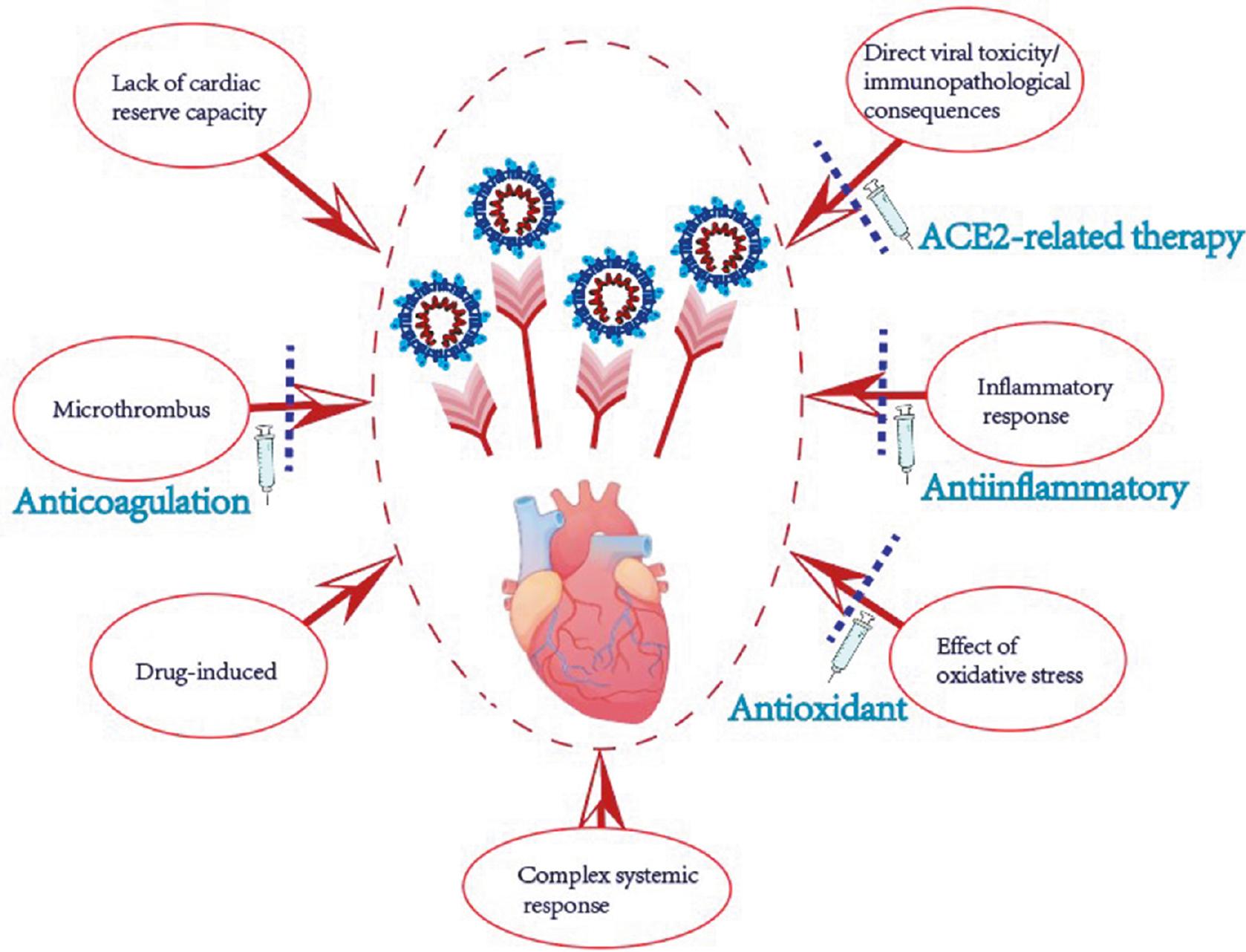
Treatment and Prevention Methods
Anticoagulation Agents
The phenomenon of thrombogenesis after COVID-19 recovery indicates anticoagulation may be necessary [83]. One review has indicated that the risk of thrombosis should be assessed for each patient as soon as possible, and anticoagulants should be provided when necessary [84] (Figure 3).
However, a retrospective cohort study has found no significant difference in overall mortality and MI incidence in patients with anticoagulation. The absence of significant differences might have been due to improper dosing and administration of anticoagulants.
Overall, anticoagulants and antiplatelets are crucial for preventing cardiovascular complications, particularly in patients with comorbidities [85].
Some data have indicated that aspirin taken within 24 h after admission or 7 days before admission is associated with a significant decrease in the risk of mechanical ventilation, ICU admission, and in-hospital mortality, thus also demonstrating the importance of early cardiac intervention therapies in patients with COVID-19 [86].
Anti-Inflammatory Agents
IL-1or IL-6 Blockers
The cardiac toxicity of IL-1 has been widely recognized [87]. IL-1 blockade has shown encouraging results in similar clinical settings.
A post-hoc analysis of a phase III randomized trial on the IL-1 receptor antagonist anakinra has indicated improvements in 28-day survival in septic patients [88].
Canakinumab, a fully human monoclonal antibody neutralizing IL-1β, decreases the deterioration of cardiac and respiratory function in patients with SARS-CoV-2 infection with associated myocardial injury and elevated inflammation [89]. One study has evaluated the therapeutic potential of canakinumab by using state of the art in vitro confocal and ratiometric high-throughput microscopy.
However, other studies have not shown significant efficacy of canakinumab. Canakinumab has been found to have no beneficial effect on cellular Ca2+ signaling during excitation-contraction coupling [90]. Carthenizumab also does not significantly shorten recovery time in in patients with COVID-19 with myocardial injury and elevated inflammation [91].
Furthermore, other anti-inflammatory agents have positive therapeutic functions, such as anti-IL-6 antibodies.
The combination of corticosteroids with anti-IL-6 antibodies (tocilizumab) has been found to yield superior survival outcomes to standard of care treatment, as well as treatment with corticosteroids alone or in combination with anti-IL-1 therapy (anakinra) [92]. Additionally, anti-IL-6 antibodies have been demonstrated to decrease neuronal injury in a murine model of ventilator-induced lung injury [93]. However, their therapeutic effects on cardiac damage remain unclear.
Hormonal Drugs
Short-duration administration of low-dose corticosteroids may serve as a practical therapeutic option for treating COVID-19 [94]. The use of corticosteroids is likely to decrease mortality in patients with ARDS. This effect has been observed to be consistent between patients with COVID-19 and nonCOVID-19 ARDS, for corticosteroid types, and dosage [95]. Observational studies and randomized controlled trials have confirmed the beneficial effects of corticosteroids on short-term mortality and decreasing the need for mechanical ventilation [96].
Cortisol also plays a related role in cardiac injury: therapeutic effects of glucocorticoids include increasing the stability of the vascular endothelial barrier and decreasing tissue edema. The effect of glucocorticoids on decreasing vascular permeability in the injured myocardium may be an additional important factor in decreasing mortality among hospitalized patients with severe COVID-19 [97].
In addition to cortisol, other hormones such as estradiol also have positive effects: the effects of IL-6 on activated NOX2-dependent ROS production and MCP-1 upregulation are completely attenuated by 17β-estradiol [98].
Macrophage Targets
Patients with COVID-19 have a high density of macrophages in the epicardium and myocardium of the heart, thus highlighting the critical role of macrophages in cardiovascular complications in these patients [99].
Ranolazine (a selective late-sodium current inhibitor for treating chronic angina) and tofacitinib (a Janus kinase [JAK] inhibitor of rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis) have been confirmed to alleviate ROS production and apoptosis in CMs by blocking IL-6 and TNF-α expression in macrophages (tofacitinib), inhibiting the JAK/signal transducer and activator of transcription pathway in CMs (tofacitinib), or lowering ROS levels in CMs [100].
ACE2-Associated Therapy
The ACE2/Ang-(1–7)/MasR axis ameliorates cardiovascular diseases through vasodilation, anti-ventricular remodeling, anti-inflammation, anti-oxidation, and anti-myocardial fibrosis effects. SARS-CoV-2 binds ACE2 and increases the expression of ADAM17, which in turn induces ACE2 shedding [101]. Therefore, invasion of SARS-CoV-2 leads to a decline in ACE2. Consequently, ACE2 supplementation can be a therapeutic approach during COVID-19. Notably, because ACE inhibitors are a common blood pressure medication, substantial discussion and controversy have surrounded on the use of antihypertensive ACE inhibitors in patients with COVID-19. ACE inhibitors should be maintained or initiated in patients with myocardial infections, heart failure, or hypertension, and withdrawal of renin-angiotensin-aldosterone system inhibitors in patients could be harmful [102].
Antioxidant Agents
The key roles or effects of oxidative stress have been described above in detail, and the therapeutic effects of some antioxidants on cardiovascular complications among patients with COVID-19 have been validated. Intravenous infusion of vitamin C may be a simple and inexpensive approach to counter oxidative stress [49].
A retrospective cohort study has suggested that high-dose vitamin C might ameliorate cardiac injury during the COVID-19 pandemic [103].
In addition, melatonin has direct free radical scavenging activity and indirectly stimulates antioxidant enzymes, thus ameliorating myocardial damage; consequently, melatonin can be used to prevent myocardial damage during SARS-CoV-2 infection [104].
Select Antiviral Agents
Direct-acting antiviral agents are widely used in clinical settings, including Paxlovid (nirmatrelvir + ritonavir) and Azvudine [105]. Paxlovid is effective in decreasing severe COVID-19 and mortality in high-risk patients, and has appeared to be more effective in patients with cardiovascular disease [106]. The effectiveness of Azvudine has also been established [107]. In addition, patients receiving tocilizumab-remdesivir treatment show significant clinical improvements in CRP levels and the P/F ratio [108].
However, some risks are associated with use of Paxlovid in patients with heart transplants [109], and analysis of more cases is necessary to assess the drug interactions [110].
In addition to the drugs described above, ursodeoxycholic acid (UDCA) protects against SARS-CoV-2 infection by decreasing ACE2. The UDCA-mediated downregulation of ACE2 has been found to decrease susceptibility to SARS-CoV-2 infection in vitro, in vivo, and in human lungs and livers perfused ex situ [111].
Even if these drugs might potentially alleviate the disease and improve cardiac function, they would also pose a risk of cardiotoxicity and would need to be used with caution [112].
Conclusion
Myocardial injury is associated with COVID-19 severity and fatal outcomes. COVID-19 causes acute myocardial injury during the disease period or chronic myocardial injury, and acute myocarditis after recovery. The SARS-CoV-2 virus may trigger heart attacks and malignant arrhythmia by binding ACE2 and inducing inflammatory storms. The presence of comorbidities is likely to cause imbalances leading to myocardial involvement.
Notably, inflammation alone cannot explain the observed myocardial damage, such as fulminant myocarditis and arrhythmia, occurring after recovery from COVID-19. The mechanism through which COVID-19 permanently attacks the heart, either through direct injury or an unappreciated inflammatory response, warrants further study.
Clinicians should check for markers of myocardial injury in patients with COVID-19, particularly patients with advanced age, comorbidities, and inflammation. Finally, early and specific anti-inflammatory or cardio-protective therapies should be clinically administered to patients with COVID-19.