- Record: found
- Abstract: found
- Article: found
Amantadine Did Not Positively Impact Cognition in Chronic Traumatic Brain Injury: A Multi-Site, Randomized, Controlled Trial
Read this article at
Abstract
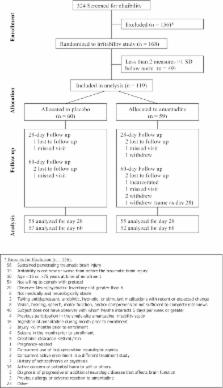
Despite limited evidence to support the use of amantadine to enhance cognitive function after traumatic brain injury (TBI), the clinical use for this purpose is highly prevalent and is often based on inferred belief systems. The aim of this study was to assess effect of amantadine on cognition among individuals with a history of TBI and behavioral disturbance using a parallel-group, randomized, double-blind, placebo-controlled trial of amantadine 100 mg twice-daily versus placebo for 60 days. Included in the study were 119 individuals with two or more neuropsychological measures greater than 1 standard deviation below normative means from a larger study of 168 individuals with chronic TBI (>6 months post-injury) and irritability. Cognitive function was measured at treatment days 0, 28, and 60 with a battery of neuropsychological tests. Composite indices were generated: General Cognitive Index (included all measures), a Learning Memory Index (learning/memory measures), and Attention/Processing Speed Index (attention and executive function measures). Repeated-measures analysis of variance revealed statistically significant between-group differences favoring the placebo group at day 28 for General Cognitive Index ( p = 0.002) and Learning Memory Index ( p = 0.001), but not Attention/Processing Speed Index ( p = 0.25), whereas no statistically significant between-group differences were found at day 60. There were no statistically significant between-group differences on adverse events. Cognitive function in individuals with chronic TBI is not improved by amantadine 100 mg twice-daily. In the first 28 days of use, amantadine may impede cognitive processing. However, the effect size was small and mean scores for both groups were generally within expectations for persons with history of complicated mild-to-severe TBI, suggesting that changes observed across assessments may not have functional significance. The use of amantadine to enhance cognitive function is not supported by these findings.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Placebo-Controlled Trial of Amantadine for Severe Traumatic Brain Injury
- Record: found
- Abstract: found
- Article: not found
Procedures for comparing samples with multiple endpoints.
- Record: found
- Abstract: found
- Article: not found