- Record: found
- Abstract: found
- Article: not found
Human T Cell Receptor γδ Cells Recognize Endogenous Mevalonate Metabolites in Tumor Cells
Read this article at
Abstract
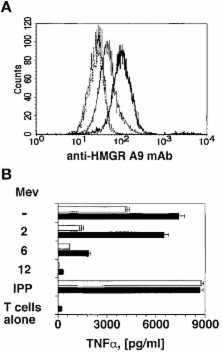
T lymphocytes expressing the T cell receptor (TCR)-γδ recognize unknown antigens on tumor cells. Here we identify metabolites of the mevalonate pathway as the tumor ligands that activate TCR-γδ cells. In tumor cells, blockade of hydroxy-methylglutaryl-CoA reductase (HMGR), the rate limiting enzyme of the mevalonate pathway, prevents both accumulation of mevalonate metabolites and recognition by TCR-γδ cells. When metabolite accumulation is induced by overexpressing HMGR or by treatment with nitrogen-containing bisphosphonate drugs, tumor cells derived from many tissues acquire the capacity to stimulate the same TCR-γδ population. Accumulation of mevalonate metabolites in tumor cells is a powerful danger signal that activates the immune response and may represent a novel target of tumor immunotherapy.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Structural mechanism for statin inhibition of HMG-CoA reductase.
- Record: found
- Abstract: found
- Article: not found
Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates.
- Record: found
- Abstract: found
- Article: not found
