- Record: found
- Abstract: found
- Article: found
New frontiers in esophageal radiology
Read this article at
Abstract
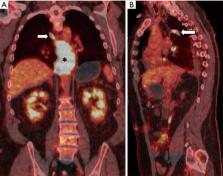
Esophageal cancer is the sixth most common cause of cancer related mortality worldwide. Advances in treatment have translated into steadily improving survival rates. Accurate preoperative staging of esophageal cancer is imperative in order to provide an accurate prognosis and direct patients to the most appropriate treatment. Current preoperative staging relies on imaging, most commonly endoscopic ultrasound (EUS), computed tomography (CT) and positron emission tomography (PET). A combination of these modalities should be used in preoperative staging, as each has advantages over another. Magnetic resonance imaging (MRI) has always shown promise in its ability to accurately stage esophageal cancer, though it has not been consistently adopted as a common tool for this purpose. Recent research has demonstrated that MRI can become an integral part of esophageal cancer clinical staging. Advances in MR technology that utilize radial sampling allow for shorter, free breathing techniques without degradation of image quality, resulting in improved capability for T and N staging of esophageal cancer. MRI enhanced with superparamagnetic iron oxide (SPIO) and ultrasmall SPIO (USPIO) nanoparticles has been shown to be useful for the detection of metastatic disease in lymph nodes. This article will review the current evidence in the role that imaging plays in staging esophageal cancer.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012.
- Record: found
- Abstract: found
- Article: not found
Preoperative chemoradiotherapy for esophageal or junctional cancer.
- Record: found
- Abstract: found
- Article: not found