- Record: found
- Abstract: found
- Article: found
Preclinical Evaluation of a Novel Lentiviral Vector Driving Lineage-Specific BCL11A Knockdown for Sickle Cell Gene Therapy
Read this article at
Abstract
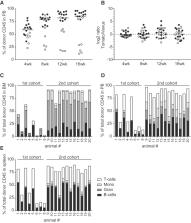
In this work we provide preclinical data to support initiation of a first-in-human trial for sickle cell disease (SCD) using an approach that relies on reversal of the developmental fetal-to-adult hemoglobin switch. Erythroid-specific knockdown of BCL11A via a lentiviral-encoded microRNA-adapted short hairpin RNA (shRNA miR) leads to reactivation of the gamma-globin gene while simultaneously reducing expression of the pathogenic adult sickle β-globin. We generated a refined lentiviral vector (LVV) BCH-BB694 that was developed to overcome poor vector titers observed in the manufacturing scale-up of the original research-grade LVV. Healthy or sickle cell donor CD34 + cells transduced with Good Manufacturing Practices (GMP)-grade BCH-BB694 LVV achieved high vector copy numbers (VCNs) >5 and gene marking of >80%, resulting in a 3- to 5-fold induction of fetal hemoglobin (HbF) compared with mock-transduced cells without affecting growth, differentiation, and engraftment of gene-modified cells in vitro or in vivo. In vitro immortalization assays, which are designed to measure vector-mediated genotoxicity, showed no increased immortalization compared with mock-transduced cells. Together these data demonstrate that BCH-BB694 LVV is non-toxic and efficacious in preclinical studies, and can be generated at a clinically relevant scale in a GMP setting at high titer to support clinical testing for the treatment of SCD.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry.
- Record: found
- Abstract: found
- Article: not found
Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1.
- Record: found
- Abstract: found
- Article: not found