- Record: found
- Abstract: found
- Article: found
Effects of Addition of Linseed and Marine Algae to the Diet on Adipose Tissue Development, Fatty Acid Profile, Lipogenic Gene Expression, and Meat Quality in Lambs
Read this article at
Abstract
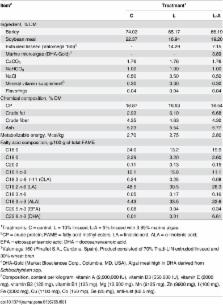
This study examined the effect of linseed and algae on growth and carcass parameters, adipocyte cellularity, fatty acid profile and meat quality and gene expression in subcutaneous and intramuscular adipose tissues (AT) in lambs. After weaning, 33 lambs were fed three diets up to 26.7 ± 0.3 kg: Control diet (barley and soybean); L diet (barley, soybean and 10% linseed) and L-A diet (barley, soybean, 5% linseed and 3.89% algae). Lambs fed L-A diet showed lower average daily gain and greater slaughter age compared to Control and L ( P < 0.001). Carcass traits were not affected by L and L-A diets, but a trend towards greater adipocyte diameter was observed in L and L-A in the subcutaneous AT ( P = 0.057). Adding either linseed or linseed and algae increased α-linolenic acid and eicosapentaenoic acid contents in both AT ( P < 0.001); however, docosahexaenoic acid was increased by L-A ( P < 0.001). The n-6/ n-3 ratio decreased in L and L-A ( P < 0.001). Algae had adverse effects on meat quality, with greater lipid oxidation and reduced ratings for odor and flavor. The expression of lipogenic genes was downregulated in the subcutaneous AT ( P < 0.05): a cetyl-CoA carboxylase 1 ( ACACA) in L and L-A and lipoprotein lipase ( LPL) and stearoyl-CoA desaturase ( SCD) in L-A. Fatty acid desaturase 1 ( FADS1), fatty acid desaturase 2 ( FADS2) and fatty acid elongase 5 (ELOVL5) were unaffected. In the subcutaneous AT, supplementing either L or L-A increased peroxisome proliferator-activated receptor gamma ( PPARG) and CAAT-enhancer binding protein alpha (CEBPA) ( P < 0.05), although it had no effect on sterol regulatory element-binding factor 1 ( SREBF1). In the intramuscular AT, expression of ACACA, SCD, FADS1 and FADS2 decreased in L and L-A ( P < 0.001) and LPL in L ( P < 0.01), but PPARG, CEBPA and SREBF1 were unaffected.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Distribution, interconversion, and dose response of n−3 fatty acids in humans
- Record: found
- Abstract: found
- Article: not found
From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions.
- Record: found
- Abstract: found
- Article: not found