- Record: found
- Abstract: found
- Article: found
Autophagy of umbilical cord mesenchymal stem cells induced by rapamycin conduces to pro-angiogenic function of the conditioned medium
Read this article at
Abstract
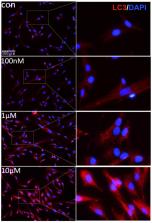
Angiogenesis is critical for wound healing and tissue repair. Umbilical cord mesenchymal stem cells (UCMSCs)-conditioned medium has certain actions to promote angiogenesis, and is expected for wound healing and tissue repair. However, recent studies showed that the pro-angiogenic efficacy of unprocessed MSCs-conditioned medium is low, and insufficient for tissue repair. Autophagy is a process for protein recycling and a contributor for cell exocrine, which may enhance pro-angiogenic efficacy of the conditioned medium by stimulating cytokine release from UCMSCs. Therefore, in this study we attempted to obtain enhanced autophagy in UCMSCs using different concentrations of rapamycin and compared pro-angiogenic functions of the conditioned media. The in vitro data showed that although 100 nM-10 μM rapamycin all could induce autophagy in UCMSCs, 100 nM was the best dose to optimize the angiogenic effect of the conditioned medium. The in vivo data also showed that pro-angiogenic effect of the optimized conditioned medium was more obvious than that of the control conditioned medium (0 nM group) in the injected matrigel plaques. Further, the expressions of VEGF, FGF-2, MMP-9, PDGF-α and PDGF-β were markedly increased in UCMSCs treated with 100 nM rapamycin. In conclusion, appropriately enhancing autophagy of UCMSC can improve pro-angiogenic efficacy of the conditioned medium, which may optimize therapeutic applications of UCMSCs-conditioned medium in wound healing and tissue repair.
Highlights
-
•
The conditional medium derived from umbilical cord mesenchymal stem cells (UCMSCs) has been used in regeneration medicine including enhancing angiogenesis, but its effect is unsatisfactory.
-
•
Autophagy has been reported to enhance exocrine functions of stem cells.
-
•
We found that inducing autophagy of UCMSCs could enhance a pro-angiogenic efficacy of the conditioned medium.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Autophagy maintains the metabolism and function of young and old (hematopoietic) stem cells

- Record: found
- Abstract: found
- Article: found
Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine
- Record: found
- Abstract: found
- Article: not found