- Record: found
- Abstract: found
- Article: not found
Multiple myeloma/hypercalcemia
Abstract
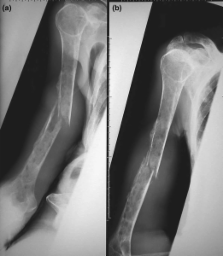
Multiple myeloma, a cancer of plasma cells, is associated with excessive tumor-induced, osteoclast-mediated bone destruction. Hypercalcemia remains the most frequent metabolic complication of myeloma in patients, and excessive osteolysis plays a major contributory role in its pathogenesis. The clinical presentation of hypercalcemia in patients varies depending on the level of ionized calcium; it can be life threatening, as in the case of hypercalcemic crisis, requiring immediate medical treatment to prevent death. During the past few years there have been exciting developments in our understanding of the pathogenesis of myeloma bone disease; in particular, key mediators of the osteoclastic bone resorption in myeloma have been identified, including receptor activator of nuclear factor-κB ligand (RANKL) and macrophage inflammatory protein-1α. There is also increasing evidence that Dickkopf 1, which has been shown to be over-expressed in myeloma patients, is also a potent stimulator of osteoclast formation and activity. Importantly, the available data suggest that RANKL is the final common mediator of osteoclastic bone resorption, irrespective of the upstream initiator molecule. This brief review presents an overview of the roles played by these mediators in inducing osteolysis in myeloma bone disease, and it discusses targeting RANKL as a potential new treatment strategy in myeloma bone disease and myeloma-associated hypercalcemia.
Related collections
Most cited references31
- Record: found
- Abstract: not found
- Article: not found
Clinical practice. Hypercalcemia associated with cancer.
- Record: found
- Abstract: found
- Article: not found
A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer.
- Record: found
- Abstract: found
- Article: not found
Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
