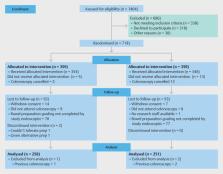
Note: To encourage dissemination of the CONSORT 2010 Statement, this article is freely accessible on the Open Medicine website and on bmj.com and will also be published in the Lancet, Obstetrics and Gynecology, PLoS Medicine, Annals of Internal Medicine, Journal of Clinical Epidemiology, BMC Medicine and Trials. The authors jointly hold the copyright of this article. For details on further use, see the CONSORT website. Randomized controlled trials, when they are appropriately designed, conducted and reported, represent the gold standard in evaluating health care interventions. However, randomized trials can yield biased results if they lack methodological rigour.1 To assess a trial accurately, readers of a published report need complete, clear and transparent information on its methodology and findings. Unfortunately, attempted assessments frequently fail because authors of many trial reports neglect to provide lucid and complete descriptions of that critical information.2-4 That lack of adequate reporting fuelled the development of the original CONSORT (Consolidated Standards of Reporting Trials) statement in 19965 and its revision 5 years later.6-8 While those statements improved the reporting quality for some randomized controlled trials,9,10 many trial reports still remain inadequate.2 Furthermore, new methodological evidence and additional experience has accumulated since the last revision in 2001. Consequently, we organized a CONSORT Group meeting to update the 2001 statement.6-8 We introduce here the result of that process, CONSORT 2010. Intent of CONSORT 2010 The CONSORT 2010 Statement is this paper including the 25-item checklist in the table (see Table 1) and the flow diagram (see Fig. 1). It provides guidance for reporting all randomized controlled trials, but focuses on the most common design type—individually randomized, 2-group parallel trials. Other trial designs, such as cluster randomized trials and non-inferiority trials, require varying amounts of additional information. CONSORT extensions for these designs,11,12 and other CONSORT products, can be found through the CONSORT website. Along with the CONSORT statement, we have updated the explanation and elaboration article,13 which explains the inclusion of each checklist item, provides methodological background and gives published examples of transparent reporting. Diligent adherence by authors to the checklist items facilitates clarity, completeness and transparency of reporting. Explicit descriptions, not ambiguity or omission, best serve the interests of all readers. Note that the CONSORT 2010 Statement does not include recommendations for designing, conducting and analyzing trials. It solely addresses the reporting of what was done and what was found. Nevertheless, CONSORT does indirectly affect design and conduct. Transparent reporting reveals deficiencies in research if they exist. Thus, investigators who conduct inadequate trials, but who must transparently report, should not be able to pass through the publication process without revelation of their trial’s inadequacies. That emerging reality should provide impetus to improved trial design and conduct in the future, a secondary indirect goal of our work. Moreover, CONSORT can help researchers in designing their trial. Background to CONSORT Efforts to improve the reporting of randomized controlled trials accelerated in the mid-1990s, spurred partly by methodological research. Researchers had shown for many years that authors reported such trials poorly, and empirical evidence began to accumulate that some poorly conducted or poorly reported aspects of trials were associated with bias.14 Two initiatives aimed at developing reporting guidelines culminated in one of us (DM) and Drummond Rennie organizing the first CONSORT statement in 1996.5 Further methodological research on similar topics reinforced earlier findings15 and fed into the revision of 2001.6-8 Subsequently, the expanding body of methodological research informed the refinement of CONSORT 2010. More than 700 studies comprise the CONSORT database (located on the CONSORT website), which provides the empirical evidence to underpin the CONSORT initiative. Indeed, CONSORT Group members continually monitor the literature. Information gleaned from these efforts provides an evidence base on which to update the CONSORT statement. We add, drop or modify items based on that evidence and the recommendations of the CONSORT Group, an international and eclectic group of clinical trialists, statisticians, epidemiologists and biomedical editors. The CONSORT Executive (KFS, DGA, DM) strives for a balance of established and emerging researchers. The membership of the group is dynamic. As our work expands in response to emerging projects and needed expertise, we invite new members to contribute. As such, CONSORT continually assimilates new ideas and perspectives. That process informs the continually evolving CONSORT statement. Over time, CONSORT has garnered much support. More than 400 journals, published around the world and in many languages, have explicitly supported the CONSORT statement. Many other health care journals support it without our knowledge. Moreover, thousands more have implicitly supported it with the endorsement of the CONSORT statement by the International Committee of Medical Journal Editors (www.icmje.org). Other prominent editorial groups, the Council of Science Editors and the World Association of Medical Editors, officially support CONSORT. That support seems warranted: when used by authors and journals, CONSORT seems to improve reporting.9 Development of CONSORT 2010 Thirty-one members of the CONSORT 2010 Group met in Montebello, Canada, in January 2007 to update the 2001 CONSORT statement. In addition to the accumulating evidence relating to existing checklist items, several new issues had come to prominence since 2001. Some participants were given primary responsibility for aggregating and synthesizing the relevant evidence on a particular checklist item of interest. Based on that evidence, the group deliberated the value of each item. As in prior CONSORT versions, we kept only those items deemed absolutely fundamental to reporting a randomized controlled trial. Moreover, an item may be fundamental to a trial but not included, such as approval by an institutional ethical review board, because funding bodies strictly enforce ethical review and medical journals usually address reporting ethical review in their instructions for authors. Other items may seem desirable, such as reporting on whether on-site monitoring was done, but a lack of empirical evidence or any consensus on their value cautions against inclusion at this point. The CONSORT 2010 Statement thus addresses the minimum criteria, although that should not deter authors from including other information if they consider it important. After the meeting, the CONSORT Executive convened teleconferences and meetings to revise the checklist. After 7 major iterations, a revised checklist was distributed to the larger group for feedback. With that feedback, the executive met twice in person to consider all the comments and to produce a penultimate version. That served as the basis for writing the first draft of this paper, which was then distributed to the group for feedback. After consideration of their comments, the executive finalized the statement. The CONSORT Executive then drafted an updated explanation and elaboration manuscript, with assistance from other members of the larger group. The substance of the 2007 CONSORT meeting provided the material for the update. The updated explanation and elaboration manuscript was distributed to the entire group for additions, deletions and changes. That final iterative process converged to the CONSORT 2010 Explanation and Elaboration.13 Changes in CONSORT 2010 The revision process resulted in evolutionary, not revolutionary, changes to the checklist (Table 1), and the flow diagram was not modified except for 1 word (Fig. 1). Moreover, because other reporting guidelines augmenting the checklist refer to item numbers, we kept the existing items under their previous item numbers except for some renumbering of items 2 to 5. We added additional items either as a sub-item under an existing item, an entirely new item number at the end of the checklist, or (with item 3) an interjected item into a renumbered segment. We have summarized the noteworthy general changes in Box 1 and specific changes in Box 2. The CONSORT website contains a side-by-side comparison of the 2001 and 2010 versions. Implications and limitations We developed CONSORT 2010 to assist authors in writing reports of randomized controlled trials, editors and peer reviewers in reviewing manuscripts for publication, and readers in critically appraising published articles. The CONSORT 2010 Explanation and Elaboration provides elucidation and context to the checklist items. We strongly recommend using the explanation and elaboration in conjunction with the checklist to foster complete, clear and transparent reporting and aid appraisal of published trial reports. CONSORT 2010 focuses predominantly on the 2-group, parallel randomized controlled trial, which accounts for over half of trials in the literature.2 Most of the items from the CONSORT 2010 Statement, however, pertain to all types of randomized trials. Nevertheless, some types of trials or trial situations dictate the need for additional information in the trial report. When in doubt, authors, editors and readers should consult the CONSORT website for any CONSORT extensions, expansions (amplifications), implementations or other guidance that may be relevant. The evidence-based approach we have used for CONSORT also served as a model for development of other reporting guidelines, such as for reporting systematic reviews and meta-analyses of studies evaluating interventions,16 diagnostic studies,17 and observational studies.18 The explicit goal of all these initiatives is to improve reporting. The Enhancing the Quality and Transparency of Health Research (EQUATOR) Network will facilitate development of reporting guidelines and help disseminate the guidelines: www.equator-network.org provides information on all reporting guidelines in health research. With CONSORT 2010, we again intentionally declined to produce a rigid structure for the reporting of randomized trials. Indeed, SORT19 tried a rigid format, and it failed in a pilot run with an editor and authors.20 Consequently, the format of articles should abide by journal style, editorial directions, the traditions of the research field addressed, and, where possible, author preferences. We do not wish to standardize the structure of reporting. Authors should simply address checklist items somewhere in the article, with ample detail and lucidity. That stated, we think that manuscripts benefit from frequent subheadings within the major sections, especially the methods and results sections. CONSORT urges completeness, clarity and transparency of reporting, which simply reflects the actual trial design and conduct. However, as a potential drawback, a reporting guideline might encourage some authors to report fictitiously the information suggested by the guidance rather than what was actually done. Authors, peer reviewers and editors should vigilantly guard against that potential drawback and refer, for example, to trial protocols, to information on trial registers and to regulatory agency websites. Moreover, the CONSORT 2010 Statement does not include recommendations for designing and conducting randomized trials. The items should elicit clear pronouncements of how and what the authors did, but do not contain any judgments on how and what the authors should have done. Thus, CONSORT 2010 is not intended as an instrument to evaluate the quality of a trial. Nor is it appropriate to use the checklist to construct a “quality score.” Nevertheless, we suggest that researchers begin trials with their end publication in mind. Poor reporting allows authors, intentionally or inadvertently, to escape scrutiny of any weak aspects of their trials. However, with wide adoption of CONSORT by journals and editorial groups, most authors should have to report transparently all important aspects of their trial. The ensuing scrutiny rewards well-conducted trials and penalizes poorly conducted trials. Thus, investigators should understand the CONSORT 2010 reporting guidelines before starting a trial as a further incentive to design and conduct their trials according to rigorous standards. CONSORT 2010 supplants the prior version published in 2001. Any support for the earlier version accumulated from journals or editorial groups will automatically extend to this newer version, unless specifically requested otherwise. Journals that do not currently support CONSORT may do so by registering on the CONSORT website. If a journal supports or endorses CONSORT 2010, it should cite one of the original versions of CONSORT 2010, the CONSORT 2010 Explanation and Elaboration, and the CONSORT website in their “Instructions to authors.” We suggest that authors who wish to cite CONSORT should cite this or another of the original journal versions of CONSORT 2010 Statement, and, if appropriate, the CONSORT 2010 Explanation and Elaboration.13 All CONSORT material can be accessed through the original publishing journals or the CONSORT website. Groups or individuals who desire to translate the CONSORT 2010 Statement into other languages should first consult the CONSORT policy statement on the website. We emphasize that CONSORT 2010 represents an evolving guideline. It requires perpetual reappraisal and, if necessary, modifications. In the future we will further revise the CONSORT material considering comments, criticisms, experiences and accumulating new evidence. We invite readers to submit recommendations via the CONSORT website. Figure 1 Flow diagram of the progress through the phases of a parallel randomized trial of 2 groups Box 1 Noteworthy general changes in CONSORT 2010 Statement Box 2 Noteworthy specific changes in CONSORT 2010 Statement Table 1 CONSORT 2010 checklist of information to include when reporting a randomized trial