- Record: found
- Abstract: found
- Article: found
Cortical Networks Relating to Arousal Are Differentially Coupled to Neural Activity and Hemodynamics
Read this article at
Abstract
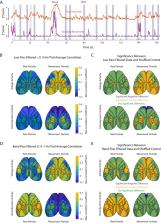
Even in the absence of specific sensory input or a behavioral task, the brain produces structured patterns of activity. This organized activity is modulated by changes in arousal. Here, we use wide-field voltage imaging to establish how arousal relates to cortical network voltage and hemodynamic activity in spontaneously behaving head-fixed male and female mice expressing the voltage-sensitive fluorescent FRET sensor Butterfly 1.2. We find that global voltage and hemodynamic signals are both positively correlated with changes in arousal with a maximum correlation of 0.5 and 0.25, respectively, at a time lag of 0 s. We next show that arousal influences distinct cortical regions for both voltage and hemodynamic signals. These include a broad positive correlation across most sensory-motor cortices extending posteriorly to the primary visual cortex observed in both signals. In contrast, activity in the prefrontal cortex is positively correlated to changes in arousal for the voltage signal while it is a slight net negative correlation observed in the hemodynamic signal. Additionally, we show that coherence between voltage and hemodynamic signals relative to arousal is strongest for slow frequencies below 0.15 Hz and is near zero for frequencies >1 Hz. We finally show that coupling patterns are dependent on the behavioral state of the animal with correlations being driven by periods of increased orofacial movement. Our results indicate that while hemodynamic signals show strong relations to behavior and arousal, these relations are distinct from those observed by voltage activity.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
The brain's default network: anatomy, function, and relevance to disease.
- Record: found
- Abstract: found
- Article: not found
Functional connectivity in the motor cortex of resting human brain using echo-planar MRI.
- Record: found
- Abstract: found
- Article: not found